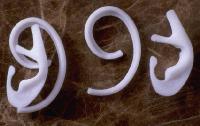Otology-5
ACOUSTIC NEUROMA AND HEARING LOSS
Introduction:
This manuscript serves to review the evidence-based literature on hearing loss in acoustic neuroma (AN) patients. The focus will be on the natural history of acoustic neuroma and how hearing progression may be influenced by factors such as tumor growth.
Historical Perspectives:
The first documented case of AN was recorded in 1777 by Dr. Sandifort. The mortality rate associated with surgery was alarmingly high at that time up to 80% due to delayed diagnosis and primitive instrumentation. Major progress in the surgical management of AN was made during the early twentieth century owing to the contribution of three surgeons. Dr. Harvey Cushing employed meticulous dissection and hemostasis, successfully lowering surgical mortality to 4%. His student, Dr. Walter Dandy, further refined technique with use of vessel clips and ligatures. Dr. Dandy was also the first surgeon to perform a complete resection of AN. Dr. William House introduced operating microscope and surgical drills to revitalize the once-condemned translabyrinthine approach in the 1960s. In addition, Dr. House introduced the concept of combining the expertise of neurosurgeons and otologists in the management of AN patients. The current state-of-the-art micro-instrumentation and intraoperative neural monitoring have enabled surgeons to achieve complete tumor removal with a very low mortality rate (<2%).
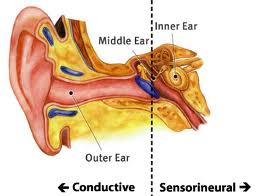
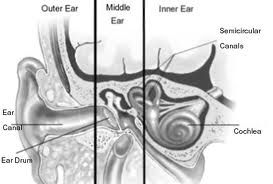
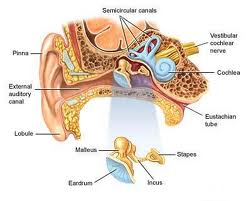
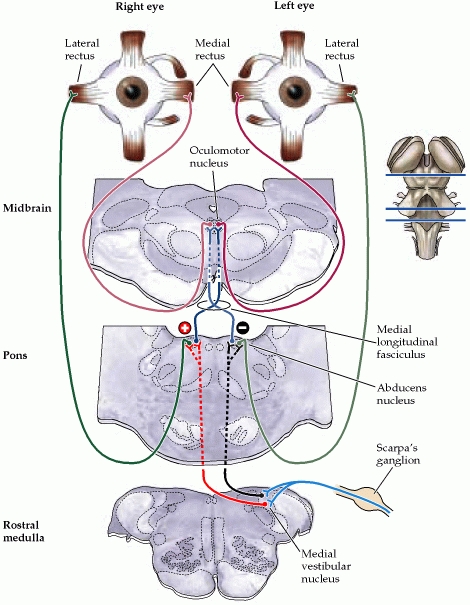
Anatomy of the Cerebellopontine Angle (CPA):
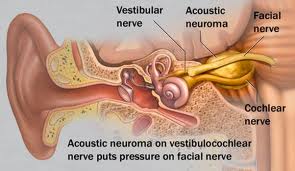
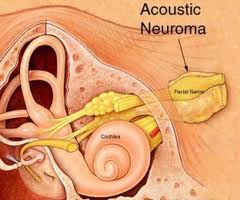
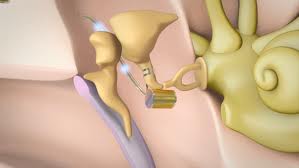
The cerebellopontine angle is a rather small area located in the posterior fossa near the origins of several vital cranial nerves. The medial and lateral borders of this space are the brainstem and the petrous portion of the temporal bone, respectively. Superiorly, it is bounded by the middle cerebellar peduncle, and inferiorly by the arachnoid tissue of the lower cranial nerves. Posteriorly, the cerebellar tonsil limits this space, as does the clivus anteriorly. Within this space lie several cranial nerves, including portions of VII – XII, as well as the CSF of the quadrimenal cistern, the arachnoid tissue of the above cranial nerves, and several blood vessels, most notably the anterior inferior cerebellar artery.
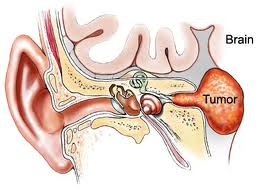
Acoustic Neuroma:
Epidemiology:
AN represents about 6% of all intracranial tumors and 80-90% of all CPA masses. Its incidence in the States is about 10 cases per million every year. It predominantly presents in adulthood. There are two forms of AN: 1) the sporadic form represents about 95% of all ANs and it presents unilaterally; 2) Neurofibromatosis type 2 (NF-2) has a strong genetic predisposition on chromosome 22, presenting as bilateral ANs. There is currently no known gender and race predilection in the development of AN. AN is a benign tumor arising from the vestibular nerve (hence it is also known as vestibular schwannoma). Majority of ANs arise from the internal auditory canal (IAC).
Hearing in AN patients:
Hearing loss is the most common symptoms of AN and can be found in about 95% of AN patients. Classically, AN patients present with asymmetric sensorineural hearing loss (SNHL) at high frequencies (down-sloping). Speech discrimination scores (SDS) typically decrease out of proportion of pure tone thresholds (PTT).
According to the American Academy of Otolaryngology-Head and Neck Surgery classification published in 1995, patients falling into categories of either class A (>70 % SDS and < 30 dB PTT loss) or B (>50% SDS and < 50 dB PTT) have serviceable hearing. Hearing of Class C (> 50% PTT and >50% SDS) and class D (< 50% SDS) patients are considered non-serviceable.
The etiology of hearing loss in AN is not known. Some proposed mechanisms include compression on the cochlear nerve, vascular disruption of the internal auditory artery, and biochemical changes in inner ear fluids.
The importance of following hearing progression in AN patients cannot be overstated. Preoperative hearing status has been shown to be the most important predictor for the success of hearing preservation surgery (middle fossa or retrosigmoid approach). A number of studies addressing the relationship between hearing progression, tumor size, and tumor growth are described in the following sections.
Tumor size:
AN can be classified by size according to the Jackler’s system: 1) Intracanalicular; 2) small < 10 mm; 3) medium 11-25 mm; 4) large 25-40 mm; 5) giant > 40 mm.
It was once thought that tumor size might be correlated with hearing progression in AN. Lustig identified a higher proportion of smaller tumors in the normal hearing group compared to his overall AN group. However, he found that hearing could be normal even in larger tumors > 3 cm. A study by Stipkovits also failed to identify any relationship between hearing and tumor size. To date, there exists no conclusive correlation between tumor size and change in hearing threshold in AN patients.
Tumor Growth:
Four phases of tumor growth have been described: intracanalicular, cisternal, compressive, and hydrocephalic. Generally, patients in intracanalicular phase display unilateral loss of vestibular or cochlear nerve function, such as hearing loss, vertigo, tinnitus, and disequilibrium. These symptoms progress as tumor grows and expands into the CPA cistern. Cranial neuropathies especially CN V and VII typically occur late in the compressive phase. Lastly, obstruction of the fourth ventricle results in hydrocephalus and can be seen in patients with visual changes and altered mentation.
Numerous papers have addressed the natural history of AN by following tumor growth in patients in the observation, or wait-and-scan group. Despite the retrospective nature and intrinsic selection bias in most of these studies, they illustrate similar results that roughly 50% of ANs grow, 40-50% remain in same size, and less than 10% involute.
The relationship between tumor growth rate and hearing was explored by Massick et al., who found a positive correlation between changes in tumor volume, pure tone average as well as speech discrimination score.
An understanding of tumor growth rate is instrumental for the clinical management of AN. Walsh demonstrated that the risk of loss of serviceable hearing in the observation period is much higher in the tumor growth group (80%) compared to the non-tumor growth group (14%). Undoubtedly, the current management of AN – observation, surgery, and radiation therapy – all hinges upon the concept of tumor growth rate. A non-growing AN that is associated with stable or minimal change in hearing is likely to be observed, while the fast growing ones with progressive deterioration of hearing would be resected or irradiated. Most integral to the success of radiation as a treatment of AN is to arrest tumor growth, which occurs in no more than 10% after radiation. Various studies have attempted to identify predictors for growth of AN without much success. There was no correlation between growth rate and patient age, gender, initial volume, and side of tumor. While potential biomarkers such as fibroblast growth factor receptor may provide an answer to tumor proliferation in the near future, the only reliable means to estimate tumor growth at present is serial MRI scanning as described below.
Diagnosis and assessment of AN:
Timely diagnosis of AN is critical because treating smaller tumors either by surgery or radiation carry a smaller risk of morbidity and higher chance of hearing preservation compared to larger tumors. While the frequencies and patterns of symptoms in AN have been thoroughly described in the literature, we must also be aware of the variations and often insidious disease progression. Patients often ignore the early and non-specific symptoms of hearing loss (~ 94%), vertigo (~39%), disequilibrium (~ 56%), or tinnitus (~64%), resulting in the delay of diagnosis. A complete otolaryngologic and neurologic exam must be performed. A high index of suspicion should be given to those with progressive unilateral SNHL, unilateral audiovestibular symptoms, facial or trigeminal nerve dysfunction, and sudden SNHL.
As described above, AN patients typically present with asymmetric SNHL with decreased SDS. Therefore, a thorough audiometric assessment is a crucial first-step in diagnosing AN. Audiogram may also demonstrate features of loss of acoustic reflex and acoustic reflex decay that are associated with retrocochlear pathology. The diagnostic efficiency of Auditory Brainstem Response (ABR) has been studied extensively. The sensitivity of ABR has been reported to be > 95% in large tumors > 3 cm. However, the false positive rate has also been described to be as high as 33 % in intracanalicular AN and false positive rate > 80%. More importantly, the response of interaural latency difference for wave V (IT5) is absent almost half the time in large tumors > 2 cm. Because of these limitations, studies on a new technique called Stacked ABR are currently underway to improve its diagnostic efficiency. Otoacoustic emissions (OAE) and vestibular testing (eg. Electronystagmography, rotatory testing, posturography) have little role in the diagnosis of AN because of low sensitivities and specificities.
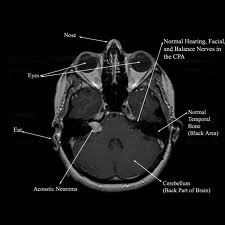
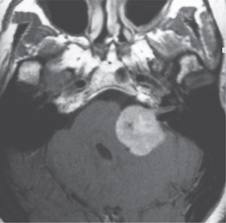
Gadolinium-enhanced MRI remains the gold standard in the diagnosing AN. It can detect tumors as small as 1 mm and differentiate AN from many CPA lesions based on its density and enhancement on T1 and T2 images. Imaging on temporal bone lesions has been previously described by Cowan et al. in our Grand Rounds Series and will not be discussed here. For those patients unsuitable for MRI scanning (eg. Metallic implant), a contrast-enhanced CT can be obtained, though it cannot reliably detect smaller tumors < 1.5 cm.
ADULT SNHL: AIDS AND ASSISTIVE DEVICES
Introduction
Hearing loss is a very common problem. Approximately 28 million people in the United States suffer from hearing loss. Several authors have demonstrated that people with hearing loss suffer significant emotional, social, and communication dysfunction. Other studies have shown that people who use hearing aids had a significant improvement in social and emotional function, communication and cognitive function, and depression over those who do not.
Although hearing aids of many types have been available for hundreds of years many people still do not use them due to many factors including education about hearing aids, cost, cosmesis, failure to accept hearing loss, poor experience with prior use and many more. Only an estimated 4.5 million Americans use hearing aids. Of those that own hearing aids 12% report never using them and of those that do wear them only 58% report being fully satisfied. As hearing aid technology advances hopefully some advances will be made in increasing the use of hearing aids and patient satisfaction with them.
Types of Hearing Aids
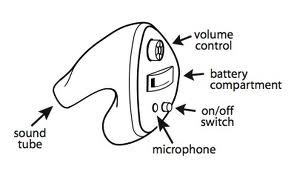
There are many types of hearing aids available today. All air conduction hearing aids have the same basic components, which include a microphone that transduces sound to electrical energy, and amplification stage, an output transducer called a receiver, and a battery to power the electronics.
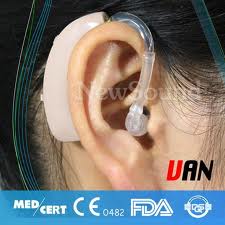
The first type is the behind the ear or BTE hearing aid. This device consists of an ear mold that sits in the concha connected to the unit that is worn behind the ear. Advantages of this type of aid are that generally the larger the device the greater the number of circuitry options available, fewer repair problems in analog devices, ability to produce more powerful amplification, the ability to use open ear molds which can benefit those in whom moisture buildup is a problem and in those with chronic otitis externa because of the ease of cleaning the ear mold, and less manual dexterity needed to insert them.
Disadvantages include large size, more noticeable, microphone in poor location for localization of sound, and loss of the benefit of using the acoustic properties of the pinna and concha. As smaller hearing aids have begun to offer more options this type has become less popular.
The second type is the in the ear or ITE hearing aid. This type of aid fits into the concha. This device is smaller than the BTE, without the unit behind the ear, but generally still offers many circuitry and venting options. The microphone is at the level of the meatus but the advantages of using the pinna and concha are still lost. This is one of the most popular hearing aid choices.
A third type of hearing aid is the in the canal or ITC type. This device fits almost completely in the external auditory canal with a small protrusion lateral to the meatus. This type provides better cosmesis than the types mentioned above and is able to take advantage of the natural influences of the pinna and concha. It requires more dexterity to use and some circuitry and venting options are not available with this type of aid.
A fourth type of hearing aid is the completely in the canal or CIC type. This relatively new type takes advantage of micro circuitry to fit all of the hearing aid components into a device that fits into the external auditory canal just lateral to the tympanic membrane. It is removed by grasping a small plastic string or wire attached to its lateral aspect. This type of aid obviously provides the best cosmesis of any type. It also is able to fully use the benefits of the pinna and concha. The medial end is within 2mm of the tympanic membrane so gain requirements are lower. Disadvantages include the need for manual dexterity to handle, some limitation in circuitry available (although this is rapidly changing), and the proximity of the microphone to the receiver making feedback a problem.
The last two types of hearing aids are used in patients with special situations. People with poor hearing in one ear and good hearing in the other ear may benefit from using a contra lateral routing of signal or CROS aid. This places a microphone at the impaired ear and routes the signal via a wire or radio signal to the other ear where signal output occurs. This allows the hearing ear to receive signals from the other side. This device prevents the head shadow effect which is the decrease in signal presented to one side of the head when it is measured on the opposite side of the head. In a patient with hearing loss in both ears but worse on one side may benefit from a biCROS aid. This provides amplification to the better ear as well as routing the signal from the poorer hearing ear.
Binaural amplification
Most patients with bilateral hearing loss will benefit from binaural hearing aids. The benefits of binaural amplification are binaural summation, which is hearing threshold improvement listening with two ears instead of one, binaural squelch which helps to tune out unwanted noise, and better sound localization.
Signal Processing
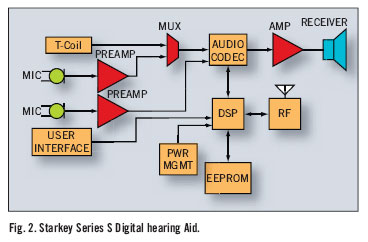
Although all hearing aids consist of the same basic component there are a wide variety of differences. The classical hearing aid is an analog device. The sound is converted to an electrical signal by the microphone, amplified and then sent to the receiver where the electrical signal is converted back to sound waves. These devices require adjustment of the hearing aid by screws or knobs on the device itself. A newer type of technology is the digitally controlled analog processor. This continues to convert sound to electric signal but has a higher range of programming options, which can be controlled from a computer or hand device. The newest devices are the digital signal processing hearing aids. These convert sound waves to numerical values for digital processing directly and generally are capable of higher fidelity and more programming options.
All hearing aids provide the benefit of gain, which is the difference in the intensity (loudness) of the input signal and the output signal at a given frequency. The problem with sensorineural hearing loss is that not only is there attenuation of signal there is also distortion of signal. The phenomenon of recruitment is associated with SNHL. This narrows the range of intensity between the level at which sound is audible to the patient and the level where the sound is uncomfortable. The distortion of signal is most often a problem for patients when there is background noise. On method of dealing with this is the use of directional instead of omni directional microphones. This allows the listener to narrow the focus of input to the hearing aid in some situations.
Older hearing aids use linear amplification of sound, which means that the ratio of input to output is one to one. This does not address the nonlinear nature of loudness growth in SNHL. This causes a problem when a high intensity sound is amplified reaching an uncomfortable level. In order to limit output a technique called peak clipping was used. This is where output is limited at a predetermined level, which causes a great deal of distortion. Newer advances have led to the technique of compression, which limits output within the dynamic range of the user in a nonlinear fashion.
Assistive listening devices
Some people with hearing loss need assistance only in special situations like talking on the telephone, watching television, listening in a classroom or hearing the doorbell ring. This need has led to the development of a host of products designed to help in particular situations. Some examples include headsets or earpieces worn to watch television, flashing lights to signal a ringing doorbell or fire alarm, or an amplified telephone. To aid in some situations a FM wireless system can be used where the speaker talks into a microphone and the output is to a headset the patient wears or directly to a personal hearing aid. This type of system is often used with children in classroom situations.
Implantable Hearing Aids
Implantable hearing aids are divided into three main categories, the temporal bone stimulators, the devices coupled to the ossicular chain, and cochlear implants. Temporal bone stimulators are the bone anchored hearing aids, which have a metallic implant directly in the temporal bone. This serves as an alternative to the classical bone conduction hearing aids which have a temporal bone stimulator worn in a head band. These devices are useful not for sensorineural hearing loss but in patients with chronically draining ears or with congenital ear malformations. They are mentioned here for completeness of hearing aid review.
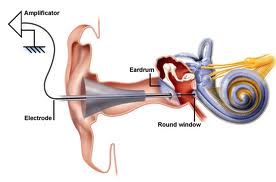
An exciting area of research and development for people with moderate to severe sensorineural hearing loss is in the area of middle ear implants, which are connected to the tympanic membrane, ossicular chain, or round window. One such device called the Vibrant Soundbridge uses an electromagnetic transducer held onto the long process of the incus connected to a magnet surrounded by a receiver coil, a demodulator package, and a conductor link, which are implanted in the skull above the mastoid. In a phase III clinical trial patients who wore this device showed improvements in satisfaction, performance, and preference over wearing their conventional hearing aids.
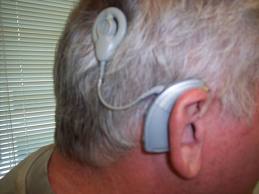
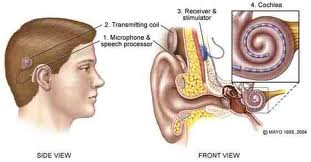
The last type of implantable hearing aid is the cochlear implant. This device is used for adults with severe sensorineural hearing loss. It consists of a microphone, a speech processor worn on the body connected to an external coil attached magnetically to an internal coil implanted on the skull, which sends the signal to an electrode, or series of electrodes implanted in the scala tympani of the cochlea. Most modern cochlear implants are have multiple electrodes to try to reproduce some of the tonotopic reception of the cochlea. Adult patients who benefit from a coclear implant generally have bilateral profound sensorineural hearing loss that have obtained no or minimal measurable benefit from a conventional hearing aid trial. Adults who are postlingually deaf, especially if they had hearing through age 6 tend to benefit most. Some prelingually deaf adults have been implanted but with poorer results.
Conclusion
Sensorineural hearing loss is a common problem among the adult population. Today there are now available a wide spectrum of hearing aids and assistive listening devices to help those with hearing deficits lead happier more productive lives. Each patient has a unique situation and set of desires when it comes to hearing. The choice of hearing aid should be tailored to the individual.
ASSESSMENT OF PERIPHERAL AND CENTRAL AUDITORY FUNCTION
The auditory pathway begins with simple interactions between mechanical sound wave energy with the pinna and results in the conscious interpretation of noise and communication. Clinical audiology has evolved new techniques and strategies to assess auditory function. It is an essential component of the otolaryngologist’s armament. The pure tone audiometry, speech audiometry, and acoustic immitance continue to be important for hearing assessment. New technologies such as ABR, EcoG, and OAEs have become the latest addition to the clinical audiologic test battery. The area of central auditory function is of relatively recent interest. Recently, efforts have been made to combine the knowledge of different specialty areas, such as otology, audiology, and speech pathology, to form a multidisciplinary approach to the diagnosis and treatment of central auditory processing disorders.
Anatomy
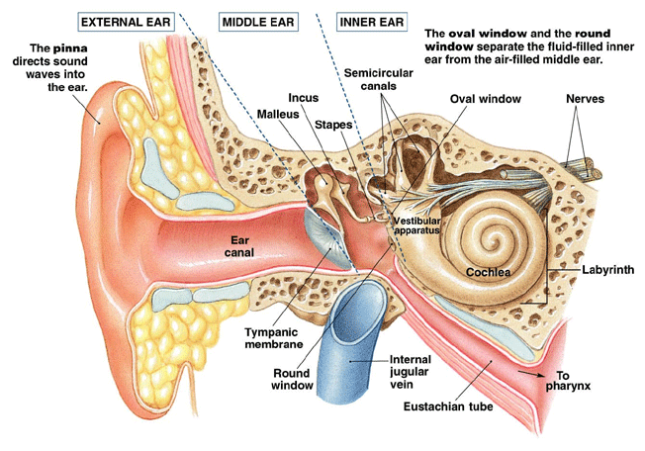
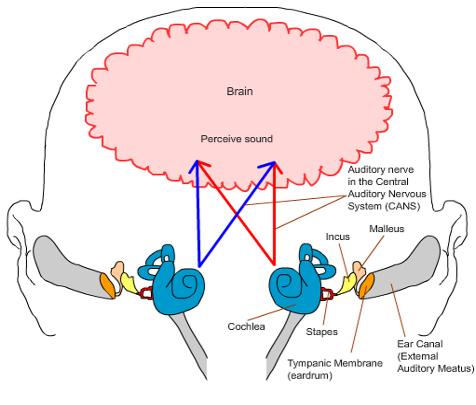
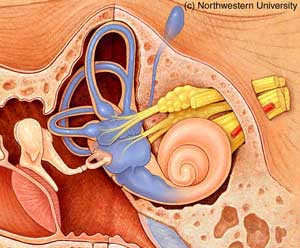
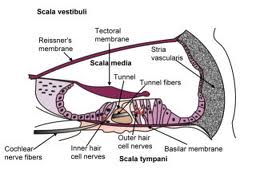
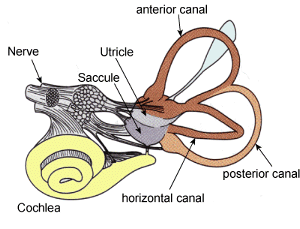
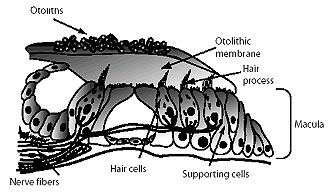
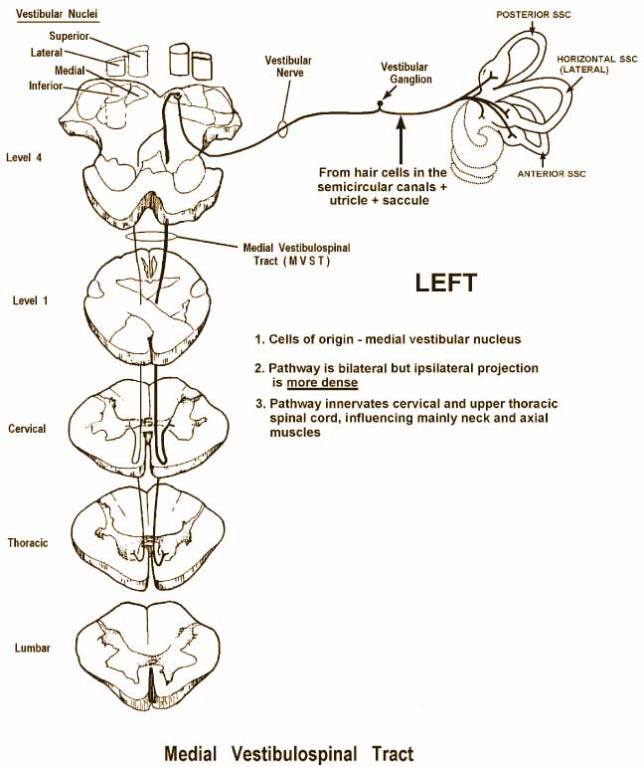
Auditory processing actually begins with the pinna and mechanical transmission through the ear canal and middle ear. As mechanical sound energy waves transmit through the tympanic membrane, ossicular chain, and oval window, a traveling wave is created through the scala vestibula with a resultant displacement of the basilar membrane and firing of cochlear hair cells. High frequency waves stimulate more proximal portions of the cochlea, while low frequency waves stimulate more distal regions of the cochlea. This neural discharge is tonotopically preserved as it travels through the eighth nerve. With low frequency sounds located ventrolaterally and high frequency sounds more dorsally. The transfer of neural impulses from the peripheral nervous system to the central nervous system occurs at the cochlear nuclei, located in the lower brainstem. The cochlear nuclei are thought to recode the auditory signal and transfer the data predominantly to the contralateral superior olivary complex through three main outputs. In addition, the cochlear nuclei pass information ipsilaterally to the lateral lemniscus. The superior olivary complex (SOC), located in the caudal portion of the pons, is thought to play an important role in binaural hearing. The SOC also appears to be an important relay station in the reflex arc of the acoustic stapedial reflex. The lateral lemniscus is considered to be the primary auditory pathway because it contains both ascending and descending pathways. The inferior colliculus is the largest auditory structure and is thought to play an important role in sound localization and frequency resolution.
On entering the thalamus, the auditory information takes two pathways to the temporal lobe. Primary auditory reception occurs in Heschl’s gyri in either hemisphere of the brain. Auditory linguistic processing occurs primarily on the left at Wernicke’s and the insular cortex. The processing of nonlinguistic information takes place primarily on the right side. Pathways from each hemisphere course to the opposite side, stimulating, inhibiting or transferring information.
Peripheral Auditory Testing
Pure tone Audiometry
Pure tone audiometry is the most common measurement of hearing sensitivity. Signals are delivered through air and bone. Pure tones are generated by an electoacoustic source that produces acoustic energy that varies as a function of cycles per second (HZ). High frequency tones initiate traveling waves whose maximum intensity will occur at the basal end of the cochlea while a low frequency tone initiates a traveling wave whose maximum intensity will occur at the apical end of the cochlea. Air conduction assesses the function of the entire auditory system from the most peripheral aspect to the central portion. Air conduction testing alone provides little information regarding the etiology of hearing loss and specific auditory pathology. Air conduction thresholds are obtained from 250Hz to 8000Hz in octave intervals. Interoctave intervals, such as 3000 and 6000Hz, are tested when successive octave thresholds differ by 20dB. When used in conjunction with bone conduction testing, they help determine both the type and severity of the hearing loss. Bone conducted sound is transmitted directly to the cochlea and is thought to be a better reflection of sensory hearing. A bone oscillator is typically placed on the mastoid process which provides an enhanced dynamic range compared with other placements such as the frontal bone.
When discussing the decibel levels in the human hearing, it is necessary to define the decibel scale that is being used. Three different units are used in audiology including dB sound pressure level (SPL), dB hearing level (HL), and dB sensation level (SL). On an audiogram the decibel scale has its reference at 0dB, which is described as audiometric 0. This is the standard for the intensity level that corresponds to the mean normal hearing threshold level, the minimal detectable intensity for each test frequency for persons with normal hearing. The human hearing extends from 20 to 20,000Hz, but the sensitivity at different frequencies varies in dB SPL. The human ear is differentially sensitive and can hear a 1000Hz tone at about 6.5dB SPL but hear a 125Hz tone at 45dB SPL and a 10,000Hz tone at about 20dB. Intensity range of human hearing extends from about 0dB HL to approximately 130dB (pain sensation). Decibels are quantified on a logarithmic basis, and the relation is described as dB = 10 log output intensity/reference intensity, and so a 1 dB increase in level signifies a ten fold increase in sound energy. Therefore, a 60dB HL is actually a million times more powerful than a 0dB HL. Sensation level is the intensity of the stimulus in decibels above an individual’s hearing threshold. For example, a word recognition test can be administered at an intensity level of 40dB SL, which is 40dB above the person’s pure tone average. The clinically normal region on an audiogram is 0 to 20dB HL. In children, hearing threshold level exceeding 15dB can be considered normal. Conversational speech is in the 40- 50db HL region; with the most significant frequencies for understanding speech being 500 through 4000Hz. Hearing sensitivity within the speech frequency region is summarized by means of calculating the pure-tone average, which are the average hearing thresholds for the 500, 1000, and 2000Hz frequencies.
Audiometric results are valid only when the patient’s responses are caused by stimulation of the test ear. ‘Crossover’ occurs when the acoustic energy presented to one ear can stimulates the non-test ear, resulting in obtained responses which represent the performance of the non-test rather than the test ear. The main mechanism of crossover is presumed to be bone conduction stimulation caused by vibration of the earphone cushion against the skull at high stimulus intensity levels. The amount that crosses over is a reflection of attenuation. The interaural attenuation of air conducted tones varies from 40 to 80dB depending on whether ear inserts or headphones are being used. Interaural attenuation values are also frequency dependent, being smaller for low frequencies and higher for high frequencies. Interaural attenuation values for bone conduction can occur even at about 0dB for bone conduction signals.
Masking is the audiometric technique used to eliminate responses by the non-test ear whenever air and bone conduction stimulation exceeds interaural attenuation. An appropriate noise is presented to the ear not being tested when the stimulus is presented to the test ear. The level of masking noise must exceed the threshold for that ear. Excess levels of masking noise must be avoided in order to prevent crossover from the masking noise.
Speech Audiometry
Speech audiometry helps determine how well a person hears and understands speech. Spondee or spondaic words are the speech stimuli used to obtain the speech reception threshold (SRT). A spondee is defined as a two syllable word spoken with equal stress on both syllables and is presented in similar fashion as pure tone audiometry. The SRT is the softest intensity level at which a patient can correctly repeat 50% of the words. SRT is measured with speech signal, and the PTA is measured within the conversational range frequency, therefore these two values should be in close agreement. An unusually good SRT relative to the PTA should alert the physician to the possibility of nonorganic hearing loss, such as malingering.
Word recognition scoring is a common clinical approach to evaluate a person’s ability to hear and understand speech. Lists of 20 to 50 words are presented to the patient at supra-threshold levels, usually 30dB above threshold. The list is phonetically balanced, which means it has speech sounds, or phonemes, that occur as often as they would in everyday conversation. Out of this list, a percentage correct is calculated. Word recognition scores of 90% or higher is considered normal while scores below this level indicate a problem with word recognition. Patients with conductive hearing loss usually show excellent word recognition. Patients with cochlear lesions have poorer discrimination. Patients with retrocochlear lesions usually have even poorer discrimination scores which sometimes may be exacerbated by the phenomenon of ‘rollover.’ Rollover is thought to occur as a result of changes in code intensity due to loss of monotonic stimulation and may be indicative of retrocochlear pathology. With ‘rollover’ the speech discrimination score goes down at higher sound intensities.
Acoustic Immittance
Immittance is a term derived from the terms for two inversely related processes for assessing middle ear function, impedance and admittance. Impedance is the resistance to the flow of acoustic energy. Admittance is the ease of which acoustic energy can flow. A middle ear with low impedence (high admittance) more readily accepts acoustic energy, whereas a middle ear with high impedence (low admittance) tends to reflect energy. Immittance is usually evaluated by tympanometry and the acoustic reflex. It is a useful diagnostic tool to identify the presence of fluid in the middle ear, evaluate Eustachian tube function, evaluate the facial nerve, and help predict audiometry.
Tympanometry reflects the mobility(compliance) of the tympanic membrane when air pressure is varied from +200 to -400dPa within the ear canal. There are three types of tympanograms: A, B, and C. A normal, or type A, tympanogram has peak compliance between 0 and -100dPa, and within a normal range of compliance. Peaks that are located within this pressure range may be overly compliant, ‘Ad,’ as with an atrophic tympanic membrane or under compliant, ‘As,’ such as with ossicular chain fixation or tympanosclerosis. A ‘type B’ tympanogram has no peak compliance and very little change in compliance with varying pressures. This pattern is most often associated with a middle ear effusion, or tympanic membrane perforations. ‘Type C’ tympanograms have a peak compliance that is located in the negative pressure ranges beyond -100dPa. This is usually seen in patients with Eustachian tube dysfunction and inadequate ventilation of the middle ear. ‘Type C’ may be a precursor to a ‘Type B’ tympanogram as the development of negative pressure precedes the presence of the effusion. The volume of air medial to the probe is also obtained with tympanometry. In general, ear canal volumes range from 0.5 to 1.0ml for children and 0.6 to 2.0ml in adults. Volume measurements greater than these may suggest tympanic membrane perforation or the presence of a patent ventilation tube.
The acoustic stapedial reflex (ASR) is defined as the lowest intensity required to elicit a stapedial muscle contraction. The neural connections for the reflex are located in the lower brainstem. However, there are influences of higher CNS structures on the reflex via the olivocochlear bundle. The afferent portion of the reflex is the ipsilateral eighth nerve to cochlear nuclei. There is a complex interaction between the ipsilateral cochlear nuclei and the bilateral motor nuclei of the seventh nerve. The efferent limb of the reflex is the seventh nerve which innervates the stapedial muscle. Contraction of the stapedial muscle tilts the anterior stapes away from the oval window and stiffens the ossicular chain and results in increased impedance which is measured as a small decrease in compliance by an ear canal probe. There are three primary acoustic reflex characteristics that are typically evaluated; 1) presence or absence of the stapedial reflex, 2) acoustic reflex threshold, and 3) acoustic reflex decay or adaptation. The time delay of the acoustic reflex is thought to be 10ms. Acoustic reflex thresholds for tones in patients with normal hearing are usually 70-80dB above their tone thresholds, and about 5dB greater for the contra lateral threshold.
Acoustic reflex decay measures the ability of the stapedius muscle to maintain sustained contraction, usually by presenting a signal 10dB above the reflex threshold for 10seconds. Even normal individuals may exhibit decay at higher frequencies, therefore the lower frequency, 500 to 1000Hz, tones are used. If the response decreases to less than half within 5 seconds, retrocochlear pathology may be suspected.
The acoustic reflex in ears with cochlear disorders is determined primarily by the degree of the sensorineural hearing loss. If auditory thresholds are below 50-55dB, the reflex thresholds are normal. If auditory thresholds are between 55- 80dB, the reflex thresholds are elevated in proportion to the increased auditory thresholds. In losses greater than 80dB, acoustic reflexes are usually absent.
Normal acoustic reflex thresholds for a broadband noise are approximately 20-25dB lower than the reflex thresholds for tones. Clinically, this is important in the evaluation of malingerers because it is not physiologically possible for the patient to truly have behavioral thresholds that are higher (worse) than acoustic reflex thresholds for tones. In patients with sensorineural loss, the difference between the broadband and tone thresholds is decreased inversely to the amount of hearing loss. Therefore, as the degree of sensorineural hearing loss in dB increases, the difference in acoustic reflex thresholds for tones and noise decreases.
The efferent limb of the reflex is explored in the diagnosis of ossicular chain disorders, such as otosclerosis and discontinuity, and facial nerve pathology. In a facial paralysis, the acoustic reflex is absent or abnormal when the recording probe is placed in the ear ipsilateral to the lesion. ASR can also be helpful in locating a seventh nerve lesion either proximal to distal to the stapedial muscle.
Eighth nerve lesions will demonstrate an absent acoustic reflex when stimuli are presented to the affected ear. An important differentiation between the acoustic reflexes in eighth nerve versus cochlear lesions is that the reflex will be absent or abnormal regardless of the degree of hearing loss, whereas in cochlear lesions it is usually dependent on the degree of hearing loss. Also, rapid reflex decay is typically found. Abnormal reflexes are also recorded when stapedial muscle function is altered by myopathic diseases such myasthenia gravis and Eaton-Lambert syndrome and hyperthyroidism.
Auditory brainstem responses
Auditory brainstem responses are impulses generated by the auditory nerve and brainstem that can be recorded on the scalp after a transient stimulus. It is not a measure of hearing in terms of the conscious perception of sound. ABRs have been detected in the human neonate as early as 25 weeks of gestation. It is not affected by sleep, sedation, or attention, and is therefore very appropriate for estimation of auditory sensitivities in infants and children who have failed conventional testing secondary to behavioral techniques.
The most widely used ABR is the click- or transient-evoked ABR. Using moderate intensity levels, an abrupt stimulus activates a large portion of the cochlea with a resultant firing of a wide range of neural frequency units. Repeatable waveforms of wave V can be detected at intensity levels within about 10dB of the average behavioral audiogram. Patterns of delay, latency, and waveform morphology can provide information about the auditory pathway from the middle ear to the lower brainstem. The click-evoked ABR is limited by its lack of frequency specificity, and may result in underestimation of the degree of hearing loss at a number of frequencies when the actual loss is sloping or unusual in shape.
Tone burst ABRs may yield more accurate results than the click-evoked ABR. Tone bursts are created with enough intensity to cause neural discharge, but at a brief enough duration to retain some frequency specificity. It is more reliable when the actual loss is sloping or unusual in shape. Latency periods are greater than for click evoked, especially at lower frequencies due to the increased travel time required to reach the more apical regions of the cochlea.
Bone conduction ABR has shown to be as reliable and repeatable as air conduction ABRs. It is particularly useful in determining whether a functional cochlea exists in structural anomalies such as canal atresia.
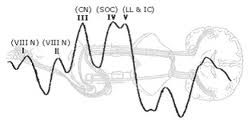
The primary goal in the evaluation of ABRs is to record a clear and reliable wave I component, which is the landmark for the remainder of the interpretation of peripheral auditory function. Wave I represents the compound action potentials from the distal end of the eighth cranial nerve. Wave II may arise from a more proximal end of the eighth nerve. Therefore, wave I and II are generated by structures that are ipsilateral to the stimulated ear. Wave III is usually prominent and is thought to arise from stimulation of the cochlear nuclei. Wave IV is likely generated by the superior olivary complex, with more contralateral than ipsilateral responses. Wave V is the most prominent and rostral component of the ABR and is thought to arise from the lateral lemniscus. When an ABR is confirmed by reproduction, absolute and interwave latencies between the individual components is measured in milliseconds. These are assessed for asymmetry, within 0.4ms between ears, and compared to normative data.
The pattern of absolute and interwave latencies can characterize different types of auditory dysfunction. Conductive hearing loss will usually demonstrate good wave morphology and normal interwave latencies, but will have a markedly delayed Wave I latency. Sensory or cochlear loss will have poor wave morphology with a weak or small and delayed Wave I. Interwave latencies will be normal. The neural type hearing loss will show a normal Wave I or overall poor morphology, interwave delayed latencies, and Wave I-III delayed latency.
ABR results differ in infants and children than those of the adult. The infant waveform shows less morphology than that of the adult. Absolute and interpeak latencies also tend to be prolonged initially but should correct by 18-24 months in patients with normal auditory development.
Electrocochleograghy
EcoG can be defined as a method of measuring stimulus-related potentials of the most peripheral portions of the auditory system. The three major components of the EcoG are the cochlear microphonic, summating potential, and the action potential. The cochlear microphonic and summating potential reflect cochlear bioelectric activity. The cochlear microphonic represents the potassium ion current flow through mainly the outer hair cells. As the stereocilia are bent away from the modiolus, the resistance decreases and flow increases. The summating potential is the DC potential recorded in the cochlea in response to sound. It may represent the shifts of outer hair cells and to a lesser extent, the inner hair cells. The action potential is generated by synchronous firing of the distal afferent eighth nerve fibers and is equivalent to ABR Wave I. The absolute amplitudes of the SP and AP vary considerably among individuals. A more common approach is to use the SP/AP amplitude ratio. Electrode placement can be in the ear canal, on the tympanic membrane, or transtympanic, which is placed on the promontory. The non-invasive placement of the electrode in the ear canal or on the tympanic membrane is
sufficient to create potentials but will have greater attenuation than the transtympanic location. Abnormal SP/AP ratio values are defined as more than 50% for the ear canal location, more than 40% for the tympanic membrane location, and more than 30% for a transtympanic location.
This examination is performed most often for intraoperative monitoring of cochlear and eighth nerve status and in the diagnosis of Meniere’s disease. Transtympanic EcoG has proven itself a reliable test to detect the presence of endolymphatic hydrops. It is theorized that hydrops affects the elasticity of the basilar membrane and contributes to the increased amplitude of the SP relative to that of the AP.
Otoacoustic emissions
Otoacoustic emissions are low energy sounds produced by the cochlea. They are thought to be acoustic byproducts of the outer hair cells, which are thought to underlie the amplification of the basilar membrane. Clinically, they are most often evoked using transient and distorted product stimulation. The evoking response causes outer hair cell motility which results in a mechanical wave that travels from the cochlea through the middle ear and tympanic membrane to the ear canal where it is recorded.
Spontaneous emissions are not present from the cochlea when there is a greater than 25dB hearing loss. Unfortunately, they are not present in all normal ears, which does not make this the test of choice to clinically assess cochlear functioning.
Transient stimuli such as clicks evoke emissions from a large portion of the cochlea. The emissions are then sampled and signal-averaged to extract them from background noise. These alternating samples are then stored in one of two memory banks and compared. Reproducibility, expressed as a percentage, is the cross correlation between these two waveforms. A reproducibility score of 50% or greater indicates that a response is present. Waveforms may vary significantly between people, but they are highly reproducible within a given individual. When hearing thresholds are better than 35dB, TEOAEs are generally present. The advantages of TEOAE are that it can separate normal from abnormal ears at 20-30dB and that it is quick. The specificity of clean, dry ears of infants is 95%. The main disadvantage is that it fails to extract responses at higher frequencies.
Distorted products are additional tones which are created when two tones, f1/lower frequency & f2/higher frequency, are presented simultaneously to a healthy cochlea. The most robust DPOAE occurs at the frequency determined by the equation 2f1-f2. Due to a nonlinear process within the cochlea, the DPOAE assesses the cochlear integrity of the region near f2. When hearing thresholds are better than 50dB, DPOAEs are generally present. The main advantage is that DPOAEs can recover OAEs above 6000Hz.
The transmission properties of the middle ear directly influence the OAE characteristics. The presence of a middle ear effusion, as in otitis media, affects both the forward and backward transmission. Although otitis media often eliminates OAEs, it is possible to record OAEs in some patients with middle ear effusion. OAE characteristics increase significantly over the first few days of life likely as a result of changes in the ear canal and middle ear. Small tympanic perforations will impede the forward transmission. This can usually be overcome with DPOAEs by increasing the amplitude.
Central Auditory Processing
There is no accepted definition of Central Auditory Processing (CAP). In its simplest form, it is what we do with what we hear. The Task Force on CAP Consensus Development defines CAP as the auditory system mechanisms and processes responsible for the following behavioral phenomena:
- Sound localization
- Auditory discrimination
- Auditory pattern recognition
- Temporal aspects of audition, including temporal resolution and masking
- Auditory performance decrements with competing and degraded acoustic signals
Deficiencies in any of these behaviors are considered central auditory processing disorders (CAPD). Results of CAPD testing have revealed clustering of test results and characteristic behaviors. These four categories are decoding, tolerance-fading memory, integration, and organization. Each of the four categories has been associated with a specific region of the brain. The Buffalo model of CAPD assessment and management takes into account the classification of CAPD as well as speech language evaluation and academic characteristics. It is important to understand that there is no one test that is sensitive enough to detect CAPD, especially in children where the variability of the tests is very wide. Therefore a battery of tests is recommended. In the Buffalo model, the CAP battery always includes the Staggered Spondaic Word (SSW) test, the Phonemic Synthesis (PS) test, a speech-in-noise (SN) test, and the masking level difference (MLD) test.
Most patients will have weaknesses in more than one category and the categories are not mutually exclusive.
Decoding
The decoding category is the most common, encompassing 50% of the weaknesses. There is an impairment of breakdown of auditory processing at the phonemic level. These patients will have difficulty accurately and quickly processing what they hear, and therefore they will need more time to figure out what they heard. They demonstrate difficulty with reading and speaking skills that depend upon phonics. This is especially noticeable when they read aloud. As children, they will often demonstrate articulation errors with the “r” and “l” sounds. Individuals of the decoding category are often compensated adequately if they have exceptional visual memory. The overlap of these deficits has suggested a relationship to the posterior temporal lobe, or Wernicke’s lobe.
The management strategies center on improvement of phonemic and metaphonemical skills. These include Hooked on Phonics and Phonemic Synthesis Program. In the academic setting, clear, concise, and repeated instructions, outlining objectives, and written instructions will benefit this category.
Tolerance-fading Memory
The second most common category is Tolerance-Fading Memory (TFM). These patients will have a poor auditory memory and poor figure ground skills, or difficulty understanding speech under adverse conditions. They often have problems with expressive language and writing. Individuals experiencing TFM typically are impulsive responders, are easily distracted and have short attention spans. They are similar to those expressed by ADHD patients. If ADD or ADHD is suspected, the Auditory Continuous Performance Test (ACPT) can be administered to screen for attention disorders. It is linked to Broca’s area of the inferior frontal lobe.
Management skills focus on improving the signal-to-noise ratio and strengthening short-term memory skills. Improving the signal-to-noise ratio can be accomplished by changing the acoustic environment of the classroom, use of assistive listening devices, and preferential seating. The use of the FM system has proved to be beneficial to this category. Noise desensitization has also shown to be beneficial. Other management strategies include use of earplugs and quiet study areas.
Integration
The Integration category includes those with difficulty integrating auditory information with other functions, such as visual and nonverbal aspects of speech. Type A patterns on the SSW are characteristic of this category. Weakness may be associated with the corpus collosum and the angular gyrus. Individuals are often labeled as dyslexics, and are usually very poor readers.
Management strategies focus on improving phonemic and metaphonologic skills. A structured phonetic approach to reading is suggested. A word processor with an audio spell-check can also be useful. Improving the signal to noise ratio is often necessary.
Organizational
The least commonly encountered category is the organizational category. It is characterized by reversals and sequencing errors. Individuals experiencing this condition are often disorganized at school and at home. It is most commonly seen secondary to another CAPD category, and is rarely found as an isolated CAPD category.
Management strategies focus on improving sequencing skills and organizational habits. Consistent routines at school and home are essential, along with checklists, appointment books, and calendars.
AURICULAR RECONSTRUCTION
Auricular reconstruction is a challenging reconstructive entity complicated by the high ratio of skin coverage to cartilage, inconsistent blood supply, and complex three-dimensional structure with subtle topographic details. The goal of reconstruction of the pinna is normal appearance, position, and symmetry with respect to the contralateral ear. Realistic expectations must be established with the patient prior to undertaking reconstruction.
In order to plan and perform a successful repair, several principles are important to apply:
- The relationship of the periauricular skin and postauricular sulcus should be preserved with reconstructive efforts.
- Thin and well-vascularized skin is a necessity. Furthermore, scar tissue, poorly vascularized tissue, and noncompliant skin must be replaced.
- The surgeon must be able to anticipate the immediate and delayed consequences of tissue manipulation. Consider the effect of tissue manipulation on hair-bearing skin, but do not allow this to compromise your ultimate quest for contralateral symmetry as hair-bearing skin can be eliminated at a secondary stage.
Psychosocial Impact
Auricular reconstruction has been shown to have a significant psychosocial benefit in the majority of patients treated, despite donor-site morbidity and a range of technical results. This was established by Horlock et al in a retrospective review. The sample group included patients with congenital or acquired auricular deformities that had either autogenous or osteointegrated reconstruction. There was significant psychosocial morbidity causing reduced self-confidence associated with auricular deformity. Teasing was prominent and the main motivation for surgery in children, while dissatisfaction with appearance was the main motivation for surgery in adults. Surgical intervention resulted in improved self-confidence, thus enhancing social life and leisure activity.
Embryology
During the sixth week of gestation, the auricle begins to arise from the first and second branchial arches. The anterior hillocks from the first branchial arch give rise to the tragus, the root of the helix, and the superior helix. The posterior hillocks from the second branchial arch give rise to the antihelix, the antitragus, and the lobule. The concha and the external auditory meatus is formed from the first branchial groove.
Anatomy
The ear is morphologically unique. The skeletal structure is composed of auricular elastic fibrocartilage which composes the upper two-thirds of the auricle. Auricular cartilage is flexible, yet it maintains form. The cutaneous coverage of the anterior-lateral surface of the ear differs from the posterior-medial surface. The anterior-lateral surface skin of the auricle lacks subcutaneous tissue and is adherent to the perichondrium. A layer of fascia containing a subdermal plexus of vessels separates the skin from the perichondrium. The posterior-medial surface skin has a deep subcutaneous fat layer that causes it to be less adherent to the cartilaginous framework.
The topographic features of the ear are incredibly important when considering reconstructive options. They are as follows:
- Helix: prominent auricular rim
- Antihelix: prominence anterior to helix
- Fossa triangularis: superior space between superior and inferior antihelical crus
- Scapha: depression between helix and antihelix
- Concha: deep cavity posterior to external auditory meatus
- Cymba conchae: portion superior to crus of helix
- Cavum conchae: portion inferior to crus of helix
- Crus of helix: beginning of helix that divides concha
- Tragus: anterior to concha and partially covering external auditory meatus
- Antitragus: posteroinferior to tragus: separated by intertragic notch
- Lobule: inferior to antitragus
The blood supply of the auricle is supplied mainly by branches of the external carotid artery which include the superficial temporal artery and the occipital artery which gives off the posterior auricular artery. The posterior-medial surface of the ear is supplied by the posterior auricular artery. The anterior-lateral surface of the ear is supplied by both the posterior auricular artery and the superficial temporal artery, creating two arterial networks. The triangular fossa and scapha are supplied by the network arising from the superficial temporal artery. The concha is supplied by the network arising from the posterior auricular artery. Consideration should be given to the blood supply when planning and designing flaps. Venous drainage is via the postauricular vein, which drains into the external jugular vein. Supplemental venous drainage flows into the superficial temporal and retromandibular veins. Lymphatic drainage of the auricle is to the preauricular, infraauricular, and mastoid lymph nodes.
The auricle has sensory innervation from the following nerves: the greater auricular nerve (C2-3), the auricular-temporal nerve (V3), the lesser occipital nerve, and a branch of the vagus nerve (Arnold’s nerve). The greater auricular nerve divides into an anterior branch, which innervates the lower half of the lateral auricle, and a posterior branch, which innervates the lower half of the medial auricle. The auricular-temporal nerve innervates the superolateral surface of the auricle. The lesser occipital nerve innervates the superomedial surface of the auricle. Arnold’s nerve innervates the concha.
The dimensions and proportions of the auricle are critical for reconstruction. The vertical height of the ear is roughly equal to the distance from the lateral orbital rim to the helical root at the level of the brow. The width of the ear is approximately 55% of its height. The helical rim protrudes between 20 and 30 degrees from the skull, which corresponds to 1 to 2.5cm. The vertical axis of the ear is tilted posteriorly (when relating the apex of the helix to the lobule) 15 to 20 degrees. The superior level of the ear is at the same height as the lateral brow. The inferior aspect of the ear is at the same height as the nasal base.
General Reconstructive Principles
Auricular reconstruction is dependent upon the defect. Auricular defects can be classified into the following categories: cutaneous and cutaneous-cartilaginous which can be full-thickness defects.
Cutaneous defects of the adherent lateral auricular surface can rarely be closed primarily. These defects are best treated with skin grafts provided there is intact perichondrium. The contralateral postauricular skin can serve as a full-thickness skin graft donor site. When lateral perichondrium is lost due to the nature of the defect, the cartilage may be removed if it is not a determinant of auricular shape, and the full-thickness skin graft can be placed on the medial perichondrium or medial skin. Medial cutaneous defects involve a more pliable skin and are often repaired by primary closure. For the same reason, medial auricular skin is an excellent donor site for a full-thickness skin graft, as previously mentioned.
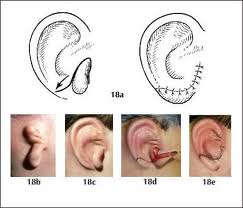
Cutaneous-cartilagenous defects may have preserved skin on one side of the defect or it may be a full-thickness defect. The main difference between this type of defect and a cutaneous defect alone is the alteration in auricular shape often caused by loss of supporting structure. Small defects may be amenable to primary closure once the defect has been converted to a full-thickness wedge excision. The decision is based on defect size and location, keeping in mind that a loss in vertical height of the auricle is inevitable. Generally, small defects in the helix or antihelix less than 0.15cm are best treated with wedge excision and primary closure. When this is performed, some central conchal cartilage must be excised to alleviate circumferential tension and prevent cupping of the auricle. Defects between 0.15cm and 2cm involving the helix or antihelix may be reconstructed by using a composite graft from the contralateral ear. In order to maintain symmetry between the auricles, the graft should be one-half the height of the defect. However, use of this technique potentially compromises the contralateral auricle, and is therefore not a first choice in reconstruction.
Many local flaps have been described for repair of full-thickness auricular loss. Basic principles of design prevail in most of the flaps ranging from chondrocutaneous advancement flaps to retroauricular island transposition flaps to tubed flaps. Vascular supply must be maintained and decreased tension with closure is necessary for viability. All flaps used to reconstruct the auricle must provide cutaneous coverage and maintain auricular structure including form and size. Regional flaps should be considered in place of local flaps when the vertical height of the auricle is decreased by more then 2cm. The most versatile regional flap used in auricular reconstruction is the temporoparietal fascia flap. This flap is often combined with an autogenous cartilage graft as a framework and can provide the required thin, highly vascular recipient site for a split-thickness skin graft.
Auricular Reconstruction Based on Defect Location
Conchal Bowl and Helical Root Defects
Conchal bowl cutaneous defects can be repaired with skin grafting. Likewise, when perichondrium or cartilage is absent, but skin remains on one side of the defect, skin grafting is used for repair. Conchal cartilage is not necessary for auricular form and can be resected without structural compromise. The retroauricular island transposition flap may be used for lateral skin and cartilage deficits. This same flap can be used in full-thickness defects involving both the medial and lateral conchal skin and conchal cartilage by bivalving the flap. Defects of the helical root can be reconstructed using a helical advancement flap which includes advancing lateral skin and cartilage down towards the deficient area.
Upper One-Third Auricular Defects
The upper one-third of the auricle can be concealed by hair to conceal a cosmetic defect. However, in many patients this portion of the ear has a functional purpose in supporting eyeglasses. Options for reconstruction of these defects include primary wound closure, full-thickness skin grafts, helical advancement flaps, retroauricular and preauricular tubed flaps, and the use of autogenous cartilage framework combined with temporoparietal fascia and split-thickness skin graft coverage. Again, the choice of the flap is dependent upon the size and location of the defect.
Middle One-Third Auricular Defects
Defects of the middle one-third of the auricle are often obvious. Small defects may be closed primarily by converting the defect into a wedge. This has a direct impact on vertical height. Some larger defects are amenable to repair with helical chondrocutaneous advancement flaps. Tubed flaps should be limited to helical reconstruction only due to the lack of a cartilaginous framework for support. Larger defects are reconstructed using a two-stage retroauricular composite flap using full-thickness retroauricular skin and autogenous cartilage. The cartilage is usually harvested from the nasal septum or contralateral or ipsilateral conchal cartilage.
Lower One-Third Auricular Defects
The lower one-third of the auricle is easiest to reconstruct due to the pliability and laxity of auricular and periauricular skin in this area. Up to half of the lobule can be resected and closed primarily with minimal deformity. The lobule can also provide tissue for advancement flaps. Reconstruction of the entire lobule is more difficult. When defects involve the entire lower one-third of the auricle, a multi-staged reconstruction involving autogenous cartilage grafting becomes necessary.
Preauricular Defects
Options fro repair of preauricular defects include primary closure, advancement flaps, and transposition flaps. Careful planning can result in the scar resting in the preauricular crease. The facial nerve should always be kept in mind when addressing these defects.
Large Auricular Defects
Auricular defects that exceed one third of the auricle are increasingly difficult to reconstruct. Multiple techniques are necessary including autogenous cartilage grafting, skin grafting, and the temporal parietal fascia flap. In certain circumstances, local skin may be adequate for coverage, but this is not the norm as the associated skin is usually absent or scarred.
Etiology
Auricular Hematoma
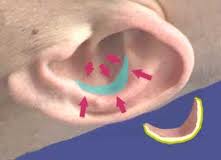
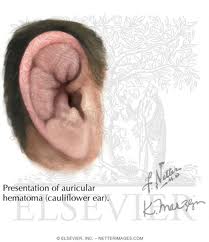
An auricular hematoma occurs due to blunt auricular trauma. If untreated or improperly treated, it will result in cauliflower ear. Auricular hematoma potentially can result in infection, cartilage necrosis, contracture, and neocartilage formation. The actual location of the hematoma has been debated and is either between the perichondrium and cartilage or intracartilaginous. A graduated treatment approach is employed dependent on the severity of the injury and the time from the initial insult. Small hematomas discovered acutely typically require needle aspiration and a bolster dressing. Aspiration is not possible a few days post-injury because the hematoma becomes a coagulated clot. After one week, the clot breaks down and aspiration is a gain possible. Larger hematomas may require an open approach or drain placement. Further treatment involves removal of all neocartilage and fibrous tissue through aggressive debridement.
Human Bites
Human bites involve the head and neck approximately 20% of the time with the ear accounting for 67% of them. The treatment goals are infection prevention and healing with good cosmesis. Human bites have a higher infection risk than bites of other mammals due to the abundant human oral flora. The infection rate in facial human bites is lower than bites in other anatomic areas due to increased vascularity in the head and neck. Recommendations have been made that treatment should be both medical and surgical including 48 hours of intravenous antibiotics and delayed surgical closure (>24 hours postinjury) to prevent infection.
Skin Cancer
The most common locations for auricular cancer are the helix, posterior surface of the ear, and antihelix. Greater than 70% of auricular skin cancer at time of presentation has an area of less than 3cm. Malignant lesions of the ear account for approximately 6% of all head and neck skin cancers.
Techniques In Auricular Reconstruction
Cartilaginous Reconstruction with Costal Cartilage
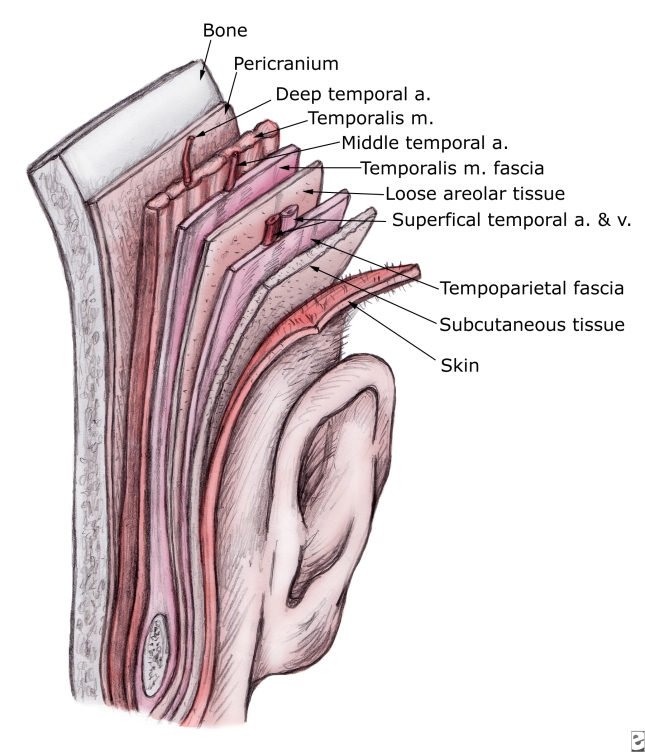
Reconstruction of the auricular framework can be performed using autogenous cartilage or alloplastic implants. Some reconstructive surgeons speculate that patients adjust better, both physically and psychologically, to reconstruction with autogenous tissue compared to alloplastic implants. Additionally, alloplastic implants have the risk of extrusion and a higher rate of infection. When patients are over 60 years old, consideration must be given to the fact that cartilage is more brittle and may be ossified depending upon location. Costal cartilage provides a reliable donor site for autogenous cartilage, specifically the sixth, seventh, and eighth rib cartilage. The synchondrosis between the sixth and seventh ribs serves as the body of the framework, while the eighth rib accounts for the helix. Determination of the size and shape of the framework is obtained by making a template from the contralateral ear. The sixth and seventh ribs are contoured to create the concha, antitragus, and curve of the antihelix. The eighth rib cartilage is freed of perichondrium on one side and contoured to form the helix. The helix is fixed to the framework and the antihelix and fossa triangularis are created using gouges. Once the cartilaginous framework has been fashioned, thin, vascular, hairless tissue capable of accepting skin grafts must be used to cover the cartilage. The temporoparietal fascia flap satisfies all of these criteria. Other advantages of the temporoparietal fascia flap include the large quantity of tissue that can be harvested (14 X 12cm) and the fascia may be transferred to the contralateral auricular region using microvascular techniques.
The temporoparietal fascia flap is composed of superficial temporal fascia which is continuous with the superficial musculoaponeurotic system (SMAS) and the deep galea. The temporoparietal fascia is deep to the skin and subcutaneous tissue. It should not be confused with the deeper temporalis fascia which surrounds the temporalis muscle. The temporoporietal fascia is 2 to 3mm thick over the parietal area and is highly vascular. The blood supply is consistent and comes from the superficial temporal artery. In order to harvest the temporoparietal fascia flap, a 6cm vertical incision is made in the scalp immediately above the auricular defect to expose the temporoparietal fascia. Elevation of the flap should be performed in the loose connective tissue or areolar tissue which is between the temporoparietal fascia and the temporalis fascia. If this plane is maintained, it is deep to the hair follicles and will avoid alopecia. The vascular pedicle of the flap is identified and protected. The frontal branch of the facial nerve is the anterior limitation of flap elevation. The posterior aspect of the flap is elevated to the posterior branch of the superficial temporal artery. The flap is rotated 180 degrees in an arc that is rotated superiorly to inferiorly so that the lateral surface of the flap lies medially along the defect. The edges of the flap are tucked under the existing skin edges. Split-thickness skin grafts are then applied to the flap. Optimal drainage is supplied by a suction drain. The temporoparietal fascia flap often obliterates the supraauricular sulcus. As a result, a second stage procedure is often necessary to recreate this sulcus.
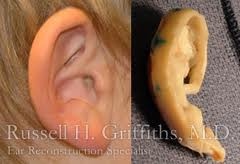
Biomaterials
Reconstruction of the total external ear has two major approaches- alloplastic prosthesis implantation and autogenous cartilage grafts. The advantages of alloplastic implants include widespread availability, consistent predetermined shape, and shortened operating time. The disadvantages are increased risk of infection, extrusion, biocompatibility, and uncertain long-term durability. To counteract some of the disadvantages, tissue engineering is being investigated using predetermined biodegradable polymers and cell isolates. Additional advantages include minimized donor site morbidity, precise creation of a complex structure, donor tissues identical to recipient tissue, and the potential for implant growth.
Porous Polyethylene Implant (Medpor)
There are multiple alloplastic auricular implants including silicone, polypropylene, and polyethylene. The porous polyethylene implant has several advantageous qualities for auricular reconstruction as it can be easily shaped, sterilized, and implanted underneath appropriate soft tissue coverage. Additionally, it is non-toxic and causes little foreign body reaction. Most importantly, this implant allows for tissue ingrowth into the material which anchors it into position and provides resistance to infection.
Microvascular Techniques
Auricular injury involving sub-total or complete amputation makes for a more complex reconstruction. Microvascular techniques have been described in complete amputations in order to avoid necrosis and distortion of auricular cartilage due to a lack of blood supply. Arterial anastomosis makes use of the primary supplying branches off of the external carotid which are the superficial temporal artery and the posterior auricular arteries. Venous anastomosis is also important and is often more difficult than arterial anastomosis. Previous literature describes of a technique with arterial anastomosis without venous anastamosis. Use of this technique emphasizes the importance of thorough debridement of non-vital tissue to allow venous channels to form between the replant and the recipient bed.
Microsurgical replantation of the ear is technically challenging, but it allows for a single procedure option for auricular reconstruction. A more natural appearing pinna usually results with this technique compared to other techniques for auricular reconstruction. Important prerequisites for successful replantation include short ischemic intervals, appropriately preserved amputated parts (in saline on ice), and compliant patients. Upon performing microsurgical replantation, secondary reconstruction options should be preserved including the postauricular skin, the temporoparietal fascia, and the main superficial temporal vessels. Small vessel caliber makes this procedure challenging. The best results can be achieved with anastomosis of both the artery and vein. However, identification of a suitable vein and venous anastomosis is especially difficult. The necessity of venous repair has been questioned for ear replants. Studies have demonstrated that venous connections form in one week through neovascularization. It is the belief of several surgeons that failure of ear replantation without venous anastomosis is due to inadequate debridement, which in turn impacts neovascularization. Additionally, wider area of contact is believed to improve neovascularization which could be provided with ear replantation by removing postauricular skin. An ear replant with venous insufficiency needs venous drainage such as leeches or skin punctures.
Nonmicrosurgical Reconstruction: Mladick and Baudet Techniques
Microsurgical techniques have been reported in the literature for auricliar reattachment, but significant complexity limits wide practice of this technique. On the other end of the spectrum, simple reattachment as a composite graft is almost certain to fail. As a result, numerous techniques have evolved to improve survival of the replanted ear. In 1971, Mladick et al proposed the principle of a retroauricular pocket for non-microsurgical replantation. The amputated part was completely deepithelialized, followed by anatomic reattachment and burial in a retroauricular pocket. A second stage procedure involved elevation of the replanted cartilage from the retroauricular pocket and split-thickness skin grafting. In 1972, Baudet et al reported a case of ear replantation where the posterior pinna skin is excised from the amputated portion of the auricle, fenestrations are made in the cartilage to allow improved vascular access to the anterior pinna skin, and a postauricular skin flap is elevated. The anterior skin is then sutured to the amputated stump and to the postauricular flap. After three months, the ear is elevated and the postauricular area is reconstructed with a split-thickness skin graft.
Venous Congestion: Leeches
Use of leeches for medicinal blood letting dates back to 200BC and remained popular well into the 19th century. Popularity waned in the late 19th century and the first 75 years of the 20th century. Modern surgical techniques including pedicled and microvascular free tissue transfer have caused the use of leech therapy to reemerge. Blood-letting allows for a temporary bypass of venous outflow obstruction until revascularization from the surrounding soft tissues will allow the flap to survive. The mechanism behind the use of leeches lies in the affect of the anticoagulant called hirudin in the saliva of the leech. Hirudin provides a prolonged decongestive effect on a tissue flap by decreasing venous engorgement, decreasing capillary pressure, and increased tissue perfusion. Due to the above properties, leech therapy can be helpful with avulsion injuries to the face where arterial blood supply is present, but venous outflow is lacking. The sparing of soft tissue provides optimal results. Leech therapy duration is based upon clinical evaluation of the involved tissue; if the tissue remains pink and viable, the leeches are no longer necessary. Once instituted, the leeches are replaced every 6 to 8 hours. Patients undergoing leech therapy should be placed on broad-spectrum antibiotics and prophylaxis against Aeromonas hydrophilia infection (second generation or greater cephalosporin, aminoglycosides, trimethoprim-sulfamethoxazol, or ciprofloxacin). Hematocrit must also be monitored in these patients. Skin punctures have also been described for venous congestion.
Antithrombotic Agent: Dextran
Dextran is a heterogeneous polysaccharide that is used after microsurgery for its antithrombotic effects on the microcirculation including alterations of platelet activity and fibrin network formation. The main advantage of dextran over other antithrombotic agents such as heparin and aspirin is the relatively lower risk of post-operative bleeding and hematoma formation. There is no clinical evidence to support the efficacy of dextran following free tissue transfer.
TPFF
The temporoparietal fascia is the most superficial fascial layer beneath the subcutaneous fat in the temporal region and is continuous with the superficial musculoaponeurotic system (SMAS) inferiorly and the galea superiorly. The superficial temporal artery supplies this area of the scalp and maintains a consistent posterior branch on which the temporoparietal fascial flap (TPFF) is normally based. The TPFF is a lateral extension of the galea and is continuous with the superficial musculoaponeurotic system of the face. It inserts on the zygoma. The TPFF has been extensively used in auricular reconstruction. The flap ranges from 2 to 4 mm in thickness and can be harvested in dimensions up to 17×14 cm. The TPFF is separate from the temporal muscle fascia, which is a thin layer of areolar tissue. The temporal muscle fascia is continuous with the pericranium above the superior temporal line. The TPFF is supplied by the STA, a terminal branch of the external carotid artery, which ascends behind the ramus of the mandible and becomes superficial 4 to 5 mm in front of the tragus. The STA lies anterior to the external ear and supplies the scalp, the external ear, face, and the parotid gland. In the majority of cases, the STA divides approximately 2 to 3 cm superior to the root of the helix into anterior (frontal) and posterior (parietal) branches. Before dividing, the artery gives rise to the middle temporal artery that supplies the temporal muscle fascia. The terminal course of the vascular pattern is variable. To protect the frontal branch of the facial nerve, the TPFF is normally raised on the posterior branch of the STA. The anterior branch is ligated approximately 3 to 4 cm from its takeoff. The distal STA arborizes over the parietal and temporal regions. The STA runs beneath the subcutaneous tissue and within the TPFF up to 12 cm above the superior attachment of the auricle. In this area of the scalp, the vessels become more superficial and anastomose with the subdermal vascular plexus. Because of the vascular architecture, this area represents the most cephalad extent of flap dissection. The superficial temporal vein runs parallel to the artery and slightly superficial to it in the majority of cases. The sensory innervation of the scalp in the area of the STA is supplied by the auriculotemporal nerve
TPFF Harvesting
The TPFF is harvested through a temporal extension of a preauricular (supratragal) facelift incision. The temporal extension should follow the curvilinear temporal line within hear bearing scalp. Dissection proceeds in a subcutaneous plane over the temporoparietal fascia to the zygomatic arch and frontal nerve. This dissection is best done sharply to avoid injury to the underlying superficial temporal vessels. The fascia is incised along the periphery of the dissection to match the dimensions of the defect. The flap can then be transposed or turned down and sutured to the periphery of the cutaneous defect. A split-thickness skin graft (usually harvested from the medial forearm or the lateral thigh) can then be applied to the TPFF.
Disadvantages of the TPFF include injury to the frontal branch of the facial nerve, hair loss from subdermal dissection, and ischemic necrosis of the distal flap if harvested beyond the temporal line.
Full-Thickness Skin Grafting
A prospective study performed in Australia between 1993 and 2002 monitored patients receiving Moh’s Micrographic Surgery for skin cancer removal followed by full-thickness skin graft repair. A total of 2673 patients were treated with the above criteria, of which 216 were auricular defects (8.1%). Eleven of these patients (5.1%) had complications including graft contracture, bleeding/hematoma, infection, and partial or complete failure.
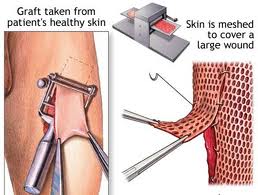
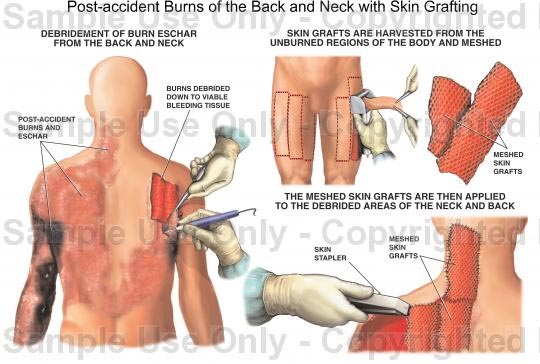
Skin Grafting
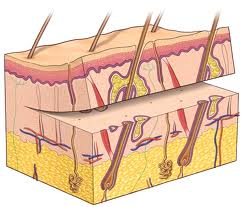
The skin graft is a fundamental reconstruction option for coverage of surgical defects. A skin graft is defined as a cutaneous free tissue transfer that has intentional separation from a donor site followed by transplantation to a recipient site. The ultimate survival of a skin graft depends upon ingrowth of capillaries from the recipient site. Keeping this in mind, avascular recipient beds including exposed bone, cartilage without perichondrium, tendon, nerve, and fascia are not ideal recipients. Skin grafts generally are used when healing by secondary intention or primary closure is not a suitable option or when skin laxity prohibits the use of a skin flap.
There are three primary types of skin grafts: full-thickness skin grafts (FTSGs), split-thickness skin grafts (STSGs) and composite skin grafts. FTSGs consist of the entire epidermis and dermis with or without small amounts of subcutaneous tissue. STSGs consist of the entire epidermis of the skin with a variable amount of dermis and are classified by thickness. Composite grafts contain tissues from two or more germ layers (skin and cartilage).
Graft survival is dependent upon the establishment of a blood supply from the recipient site. The first 24 hours involves sustaining the graft by imbibition. Approximately 48 to 72 hours after grafting vascular anastomoses between the recipient bed and donor graft begin to form in a process called inosculation. Circulation is restored to the graft within 4 to 7 days.
FTSGs are relatively easy to harvest and easy to secure to the recipient site. They tend to be more prone to necrosis than STSGs, yet FTSGs tend to contract less than STSGs. FTSGs are excellent for the repair of defects on the nasal tip, dorsum, ala, and sidewall, as well as on the lower eyelid and ear. FTSGs should not be placed into infected wounds and smoking is a relative contraindication. Donor sites shoud be carefully chosen to match the texture, thickness, and color the recipient skin. Common donor sites for facial defects include preauricular, postauricular, supraclavicular, and calvicular areas. The recipient site must be clean and not actively bleeding. FTSGs must make direct contact with the underlying wound bed and must be immobilized in the post-operative period (typically with a bolster which should be in place for 1 week) to prevent separation of the graft from the recipient site. FTSGs are sewn into place with deep basting sutures and perimeter sutures. Complete or partial graft failure is the main complication of FTSG which results from hematoma, graft-bed contact disruption, infection, smoking, and excessive electrocoagulation of the wound base. If necrosis does occur, the tissue should not be debrided as it acts as a scaffold.
STSGs lack their innate vascular and adnexal structures. They are classified as thin, medium, and thick based on thickness. STSGs can be meshed to increase surface area coverage. They offer better survival characteristics than FTSGs due to reduced nutritional requirements. STSG should be a last resort when cosmesis is of primary concern. Additionally, STSG is the least durable form of wound closure and can experience contraction, pigment variation, and creation of an additional wound. Donor site is chosen based on desired size, the patient’s ability to care for the site, impact on patient’s activity, and cosmesis. STSGs are secured using basting and perimeter sutures. Dressings are placed to prevent shearing because if shearing of the graft occurs within the first twenty-four hours, graft failure is almost certain. Revascularization of STSGs takes about 3 to 5 days. Acute complications are identical to FTSGs. Additionally, contraction is a common and unpredictable occurrence. Composite grafts are an option in specific auricular defects with the donor site being the contralateral ear.
Complications
Standard complications with auricular reconstruction apply including infection, hematoma, scarring, and poor cosmetic outcome. When the auricular cartilage is involved in the injury or the repair, the risk of perichondritis and chondritis must be considered. Inflammation of the perichondrium or cartilage after trauma predisposes to tissue ischemia and the development of Pseudomonas infection, which may ultimately lead to suppurative chondritis. Liquefactive necrosis can then ensue leading to devastating complications. Therefore, manipulation of the cartilage should be performed carefully under sterile conditions with antibiotic prophylaxis.
Conclusion
Every ear defect is unique. Many options are available for reconstruction of auricular defects including direct closure, secondary epithelization, FTSG, composite grafts, and local flaps including direct advancement, rotational flaps, transposition flaps, and subcutaneous island flaps. Factors to consider prior to choosing a reconstructive plan include size, location, depth, medical history, smoking history, and esthetic concerns.
AUTOIMMUNE INNER EAR DISEASE
Autoimmune inner ear disease (AIED) is a relatively new etiologic mechanism of sudden or rapidly progressive hearing loss which has been added to the previously accepted triad of viral, vascular, and membrane rupture. This condition implies that inner ear proteins are recognized immunologically as foreign or non-self. It has been proposed that this occurs as a primary autoimmune phenomenon, or as a result of the exposure of an immune privileged antigen through trauma or an inflammatory process. Because these inner ear antigens are not yet known, some clinicians still doubt the existence of AIED. No one can deny, however, that a large percentage of patients with suspected AIED, who were once thought to have progressive, irreversible hearing loss, will benefit from treatment with immunosuppressive drugs if they are started early in the disease course.
McCabe first described AIED in 1979. He reported on eighteen patients with bilateral rapidly progressive sensorineural hearing loss (SNHL) for which no identifiable cause could be found. Evidence of autoimmunity in these patients included a positive lymphocyte inhibition test. In this test, patient’s serum was combined with antigens derived from the membranous labyrinth of patients who had undergone translabyrinthine resection of acoustic tumors. All patients’ sera reacted to some antigens while controls did not. Additional evidence of autoimmune etiology was indicated by the fact that patients had substantial hearing improvement with steroid treatment. Further more, histopathologic examination of one patient’s temporal bone revealed vasculitis. McCabe proposed that the diagnosis of AIED be based on positive immune laboratory tests and beneficial treatment response. He also further characterized AIED from other causes of sudden sensorineural hearing loss (SSNHL) in that hearing loss is typically bilateral and asymmetric, progressive of several months with episodes of improvement, and associated with vestibular symptoms.
Since McCabe’s first description AIED, support for the existence of this disease has grown as a result of extensive basic science research. Animal studies have proven that the inner ear does in fact have immunity and is not an immune privileged site as once thought. The endolymphatic sac has been shown to contain immunocompetent cells even in a normal resting state and seems to be the inner ear site where antigen processing is carried out. It serves to protect the rest of the inner ear from foreign substances and infectious agents. Experiments have shown brisk immune mediated inflammatory responses in the endolymphatic sac after direct inoculation with viral components with preservation of hearing and balance. The cochlea on the other hand is devoid of immune cells. It has been shown, however, that when an antigen is injected into the scala tympani, it will eventually diffuse into the endolymphatic sac where it may potentially illicit an immune response.
Experimental evidence has also supported the existence of AIED. It has been found that animals immunized with inner ear proteins can produce modest, transient hearing loss and cochlear inflammation (Orozco et. al, 1990). Despite this evidence from animal models, the existence of a specific autoimmunity within the inner ear remains controversial. Although candidate antigens including a 68 kDa protein, type II collagen, and several other antigens have been proposed, a single target antigen of autoimmunity has been elusive. This is not surprising considering the fact that the inner ear is comprised of thousands of different proteins, many of which are intimately involved with auditory and vestibular functions.
Histopathology consistent with autoimmune disease is one of the criteria use to establish an autoimmune etiology. This is of course impossible to obtain with respect to the inner ear of patients with suspected AIED. A few temporal bones of patients with suspected AIED have been examined postmortem and have shown osteoneogenesis consistent with past inflammation, but the histophathology was of course not concurrent with the onset of disease.
Response to immunosuppression has often been cited as evidence for an autoimmune process in the inner ear. Through animal studies it appears that immuno-suppression can reduce immune-mediated SNHL. Animals pretreated with cyclophosphamide followed by viral challenge of the inner ear have been shown to have decreased incidence and severity of hearing loss measured by eighth nerve compound action potentials (Darmstadt et al.). We know of course, that drugs such as steroids and cytotoxic agents are not specific to immunity. These drugs can, by reducing the number of circulating leukocytes, reduce inflammation due to a number of causes.
Ultimately, we still lack the definitive evidence that most scientists and immunologists demand in order to conclude that autoimmunity does in fact occur in organ-specific manner in the inner ear.
Etiology of SSNHL
The etiology of SSNHL can be broken down into broad categories: (1) viral and infectious, (2) autoimmune, (3) labyrinthine membrane rupture/traumatic, (4) vascular, (5) neurologic, and (6) Neoplastic. There are multiple conditions within each of these categories that have been associated with SSNHL. The following is a partial list of reported causes of
Infectious
- Meningococcal meningitis
- Herpesvirus (simplex, zoster, varicella, cytomegalovirus0
- Mumps
- Human immunodeficiency virus
- Lassa fever
- Mycoplasma
- Cryptococcal meningitis
- Toxoplasmosis
- Syphilis
- Rubeola
- Rubella
- Human spumaretrovirus
Autoimmune
- Autoimmune inner ear disease (AIED)
- Ulcerative colitis
- Relapsing polychondritis
- Lupus erythematosus
- Polyarteritis nodosa
- Cogan’s syndrome
- Wegener’s granulomatosis
Traumatic
- Perilymph fistula
- Inner ear decompression sickness
- Temporal bone fracture
- Inner ear concussion
- Otologic surgery (stapedectomy)
- Surgical complication of nonotologic surgery
Vascular
- Vascular disease/alteration of microcirculation
- Buerger’s disease
- Vascular disease associated with mitochondriopathy
- Vertebrobasilar insufficiency
- Sickle cell disease
- Cardiopulmonary bypass
Neurologic
- Multiple sclerosis
- Focal pontine ischemia
- Migraine
Neoplastic
- Acoustic neuroma
- Leukemia
- Myeloma
- Metastasis to internal auditory canal
- Meningeal carcinomatosis.
Autoimmune
In addition to the proposed organ-specific AIED, autoimmune hearing loss may also be associated with or part of systemic autoimmune diseases such as Cogan’s syndrome, Wegener’s granulomatosis, polyarteritis nodosa, temporal arteritis, Buerger’s disease (thromboangitis obliterans), and systemic lupus erythomatosis. The pathogenesis of immune-mediated sensorineural deafness and vestibular dysfunction are unclear, but are presumed to include: vasculitis of vessels supplying the inner ear, auto-antibodies directed against inner ear antigenic epitopes, or cross-reacting antibodies.
Cogan’s syndrome (CS) is an autoimmune disease of the cornea and vestibuloauditory apparatus that was first described by Cogan, an ophthalmologist, in the 1940s. It occurs primarily in young adults (average age of onset 22-29 years) and typically presents with interstitial keratitis (IK) and Meniere’s-like attacks of vertigo, ataxia, tinnitus, nausea, vomiting, and hearing loss which develop within several months of each other. CS may also be associated with other systemic manifestations of the inflammatory process such as Takayasu’s-like or medium-sized vessel vasculitis. Approximately 10% of patients develop aortitis within weeks to years after the onset. Hearing fluctuation in CS coincides with disease exacerbations and remissions. Its course often culminates in deafness. One series reported that 12 out of 18 patients (67%) developed bilateral deafness.
The cause of CS is unknown. Microbial etiology has been suggested by some as a URI precedes nearly 40% of patients who present with this disease. Clinical parallels between syphilis and CS have led some to belief that CS may be caused by Borrelia burgdorferi. However, evidence so far has been inconclusive. There have also been links to Chlamydia species with CS and is an area of ongoing investigation. Temporal bone histopathologic studies done at autopsy of patients with CS are characterized by chronic inflammation including: infiltration of the spiral ligament with lymphocytes and plasma cells, endolymphatic hydrops, degenerative changes in the organ of Corti, and demyelination and atrophy of the vestibular and cochlear branches of the eight cranial nerve.
There is no criteria currently established for the diagnosis of CS. The general thinking is that the diagnosis requires clinical signs of both eye and inner ear inflammation. Work-up should include an audiogram and laboratory tests including CBC, ESR, and RPR. Imaging including MRI and/or CT should be done primarily to rule out cerebropontine angle tumors and other disorders. MRI may show enhancement of vestibular and cochlear structures with gadolinium.
History and Physical
Evaluation and management of rapidly progress or SSNHL should be considered medically urgent, if not an emergency. The primary goal is to rule out any treatable causes and begin treatment early in the course of the disease.
Diagnostic evaluation of the patient with sudden hearing loss begins with a thorough history and physical exam. Details of the circumstances surrounding the hearing loss and the time course of its onset should be elicited. Associated symptoms, such as tinnitus, vertigo or dizziness, and aural fullness should also be asked about. Clinical experience has shown that about one-third of patients note their hearing loss upon first awakening in the morning, and that about one-half of cases will have associated vertigo. Patients should also be questioned about previous otologic surgery, ototoxic drug use, and previous or concurrent viral or upper respiratory tract infections. Any history of trauma, straining, diving, flying, and intense noise exposure should be noted. Past medical history of other diseases associated with sudden hearing loss should also be obtained such as diabetes, autoimmune disorders, malignancies, neurologic conditions (multiple sclerosis), and hypercoagulable states. African-Americans should be asked about sickle cell disease.
A complete head and neck exam should be performed on all patients with sudden hearing loss. More often than not, the exam will be unremarkable, however, any processes such as middle ear effusions, infections, cholesteatoma, and cerumen impaction should be excluded. A thorough neurological exam including Weber and Rinne, cranial nerves, and cerebellar and vestibular testing should be performed.
Although AIED is categorized by most sources as cause of SSNHL, the clinical picture of AIED differs from other causes of SSNHL in that it usually consists of rapidly progressive (over weeks to months) rather than sudden (hours to days), bilateral, asymmetric, sensorineural hearing loss. The hearing loss tends to wax and wane with disease activity. Systemic manifestations are usually absent which distinguishes it from other known autoimmune disorders. One series found that less than 30% of patients with rapidly progressive SNHL had an associated systemic autoimmune disorder. Approximately 50% of patients will complain of dizziness. Episodic light-headedness or mild ataxia is more common than true vertigo. Also, these episodes occur multiple times daily during active disease as opposed to two or three discrete episodes per week, as is seen in Meniere’s disease. Occasionally symptoms of pressure and tinnitus can occur. The symptoms often progress over weeks or months but can also present as sudden hearing loss or protracted disease over many years. Most patients present with bilateral disease, and when dizziness is present, vestibular testing usually reveals bilateral reduced response. AIED has a slight predominance in middle-aged females, but can occur in both sexes and can begin in childhood.
Diagnosis
An audiogram (pure tone, speech, tympanometry, including stapedial reflex testing) should be performed on all patients with sudden hearing loss. The audiogram is the foundation of the diagnosis and provides prognostic information. Serial testing provides documentation of the progression or resolution of the hearing loss and response to treatment. In addition it may help exclude patients with secondary gain or with pseudohypacusis.
The following is a list of laboratory studies that can be ordered. Initial screening tests should be directed based on history and suspected conditions.
- Complete blood count (CBC)
- Erythrocyte sedimentation rate (ESR)
- C-reactive protein (CRP)
- Glucose
- Cholesterol/triglycerides
- T3, T4, TSH
- VDRL, RTA-ABS (MHA-TP)
- HIV
- Lyme titer
- Antigen-specific cellular immune tests Lymyphocyte transformation test (LTT) Western blot immunoassay
Magnetic resonance imaging (MRI) is recommended by the majority of authors for patients with asymmetric hearing loss. In one survey of 79 otolaryngologists, 38% would order imaging on the patient’s initial visit. MRI is useful in evaluating for acoustic tumors, multiple sclerosis and cerebrovascular accidents. There are some proponents of following these patients and imaging only if asymmetric hearing persists. However, Berg et al., in a series of acoustic neuromas showed that 13% presented with sudden hearing loss, and of these 23% recovered auditory function.
The diagnosis of AIED by most clinicians is based on the presence of bilateral progressive sensorineural hearing loss and response to immunosuppressive therapy. To this date, proof of a causal relationship of autoimmunity and sudden or rapidly progressive hearing loss has not been definitively established. Without definitive evidence of the existence of AIED, diagnostic and treatment studies are currently based on a presumptive diagnosis.
Hughes proposes that the two most clinically helpful tests for diagnosing AIED are the lymphocyte transformation test (LTT) and the Western blot immunoassay. These are both antigen-specific immune tests. The sensitivity and specificity for LTT are estimated to be 50-80% and 93% respectively with positive predictive values ranging from 56-73% depending on the disease prevalence in the tested population. When applied to high risk populations (patients with bilateral rapidly-progressive SNHL), the Western blot has a sensitivity of 88%, a specificity of 80%, and an overall positive predictive value of 92%. For these tests to work, high levels of circulating autoimmune antibodies are ideal. Because the disease waxes and wanes, testing should be performed during periods of disease activity and before treatment is initiated.
Currently, tests for AIED are not routinely used except at certain centers (Cleveland Clinic and the Massachusetts Eye and Ear Infirmary) and in experimental trials. The major drawback to these studies is the lack of availability. If testing is desired, samples of whole blood from patients can be mailed to the Cleveland Clinic Foundation Regional Laboratory by overnight carrier for LTT. The test costs $120.00 and results take approximately seven days.
The best theoretical test for AIED would be a test for a marker specific for AIED. Attempts have been made in this area and are promising. In 1990, Harris and colleagues published the results of studies which discovered, using Western blot, an anti-68 kDa autoantibody in the sera of patients with rapidly progressive SNHL. Since then, other studies have confirmed these findings in humans (Moscicki, 1990) and in autoimmunized animals (Orozco, 1990 and Harris, 1990). Interesting, despite the species of animals used, analysis of sera from hearing-impaired animals by Western blot revealed an antibody against an inner-ear antigenic epitope with a molecular weight of approximately 68 kDa.
Overall 22% to 89% of sera of patients with rapidly progressive SNHL will contain this antibody. Moscicki emphasizes the importance of testing patients during disease activity. In his series, 89% of patients with active disease tested positive for anti-68 kDa antibodies versus 0% in patients with inactive disease. Harris has reported a 94% specificity for test correlating results with responsiveness to therapy and disease activity. Studies by Billings and Harris are now searching for the specific antigen involved in AIED. So far they have isolated a 68 kDa protein that is ubiquitous in the inner as well as other areas of the body, and have recently reported evidence that links the 68kDa antigen with heat shock protein 70 (hsp 70), a highly inducible stress protein. Further research is needed in this area to determine the exact relationship of hsp 70 to AIED and whether it plays an important etiologic role or whether it is just a bi-product of the disease itself. Theories proposed are that (1) human hsp 70 may have a similar amino acid sequence to an infecting agent resulting in cross-reactivity or (2) that there may be a hsp 70 specific to the inner ear that is seen as foreign when it is over-expressed during times of chronic inflammation from an outside agent. Support for this theory has been shown by Gong and Yan (2002). They showed that guinea pigs immunized with homologous crude inner ear antigen (CIEAg) had significantly increased expression of hsp70 compared to controls. Trune et al. (1998) were unable to induce hearing loss with hsp 70 in guinea pigs. This same finding held true for Harris who immunized mice with the protein and found no hearing loss.
In addition to the 68 kDa/hsp 70, multiple other candidate antigens have been proposed such as type II collagen (Yoo et. al, 1982), beta tubulin (Connolly et al., 1997), 30 kDa protein, and c Raf.
Treatment
Treatment for AIED is controversial and widely varied from practitioner to practitioner. This is largely due to the lack of double-blind, prospective clinical trials on the matter. The general consensus is that steroids are effective and should be used. Most sources recommend prednisone 1mg/kg/day for 4 weeks followed by a slow taper if the patient responds. If the patient relapses on the taper, Harris recommends instituting high dose prednisone (up to 2mg/kg) and if continued recurrence occurs with tapering, a cytotoxic agent such as methotrexate (MTX) or cyclophosphamide (Cytoxan) should be instituted. Most sources recommend starting MTX at a dosage of 7.5-15 mg weekly with folic acid (as recommended by the FDA for treatment of rheumatoid arthritis). Harris recommends a higher dose of MTX: 15-25mg. If MTX is used, the steroids should be continued after starting the MTX as it takes one to two months for the prednisone sparing affects of MTX to begin. Most physicians begin with MTX as it has fewer side-effects than Cytoxan. If both prednisone and MTX are ineffective, Cytoxan should be used (100mg po bid).
Other authors such as McCabe are more in favor of starting cytotoxic drugs at the onset of the illness. He believes that Cytoxan is the preferred treatment of AIED rather than steroids because in his series he has found a higher response rate to this drug. He believes that because the diagnosis of AIED is based partially on response to therapy, fewer patients with this diagnosis would be missed.
It is important to monitor for side effects of both MTX and Cytoxan with routine monitoring of complete blood counts, platelets, LFTs, UA, and electrolytes. Those on Cytoxan should keep well-hydrated to prevent hemorrhagic cystitis. If possible, a physician (Rheumatologist) experienced in the use of these cytotoxic drugs should be consulted to assist with dosage adjustments and managing side effects.
Data on hearing response to therapy is relatively lacking with the majority of studies being retrospective. Sismanis (1997) looked at treatment with MTX and reported that 69.6% had improvement in hearing and 80% had improvement of vestibular symptoms. He supported the use of MTX particularly for long term use because of its low complication rate compared to cyclophosphamide and steroids.
In a prospective trial, Matteson (2001) treated patients with 3 weeks of high dose prednisone taper and found a 72% rate of partial hearing improvement. He included patients with bilateral Meniere’s disease and Cogan’s disease.
Moscicki (1994) emphasizes the utility of the Western blot for detection of anti-68 kDa antibodies to determine which patients will respond to treatment. In his series of 75% of patients with a positive test responded to treatment vs. 18% with a negative test.
Recently, Harris et. al (2003) looked at the affect of MTX versus placebo in maintaining the hearing improvement in patients responsive to prednisone. This was a randomized, double blinded, placebo controlled trial. He found a 57% rate of hearing improvement over all with initial treatment with prednisone. No difference was found however in hearing preservation after steroid treatment between the two groups. They concluded that MTX should no be used to maintain hearing improvement achieved with prednisone in patients with AIED.
Lasak et. al (2001) retrospectively looked at patient with presumed AIED and Western blot assay positive for a 68 kDa inner ear antigen who were treated with steroid alone or steroids plus cytotoxic agents (methotrexate, cyclophosphamide, and azathioprine). They found an over all improvement in hearing of 59%. The steroid-only responders tended to demonstrate a greater improvement in pure tone averages (14.8 vs 4.5 dB), while the cytotoxic-agent responders had a significantly greater improvement in speech discrimination (26.2 vs 6.9%). They concluded that cytotoxic medications improve discrimination even in some patients who are steroid non-responders.
Plasmapheresis (PMP) has also been proposed as a treatment for AIED (Luetje, 1989). The mechanism of action of plasma exchange involves the removal of circulating antibodies, antigens, and immune complexes and may enhance the effects of immunosuppressive agents. Luetje (1997) reported on his experience treating 21 patients with rapidly progressive SNHL with PMP. Several of his patients had dramatic improvement in hearing after treatment. Some were able to be taken off immunosuppressive agents and remained asymptomatic off of them. Luetje confesses that data from his series is confounded by several factors including retrospective analysis and concurrent use of immunosuppressive agents in some patients while not in others (those who could not tolerate them). Further research into this area is probably warranted. If hearing loss is truly autoimmune in some patients, it would make sense to see benefits with PMP. Perhaps effects on patients with positive screening for specific antigens such as hsp 70 would be more dramatic as is seen with steroid treatment in these patients.
It is important to keep in mind that some patients with AIED will not improve on treatment or progress to bilateral profound hearing loss. These patients are excellent candidates for cochlear implantation or other cochlear prosthetic devices and should be counseled about such options depending on the severity of their hearing loss.
Prognosis
The natural history of AIED is not known, however, clinical experience reveals that the disease waxes and wanes. Published series looking at patients with rapidly progressive SNHL and SSNHL report spontaneous recovery rates ranging from 47% to 63%. These reviews combined patients with partial and complete recovery and patients with all audiogram types. Four variables have been shown to affect recovery from SSNHL: (1) time since onset, (2) audiogram type, (3) vertigo, and (4) age. In 1984, Byl published a prospective study conducted over 8 years that evaluated 225 patients with SSNHL. Factors evaluated included age, tinnitus, vertigo, audiogram pattern, time elapsed from onset of hearing loss to initial visit, and ESR level with respect to recovery. His findings were as follows:
- Time since onset – His study confirmed that the sooner the patient was seen and therapy initiated, the better the recovery. 56% of patients presenting within the first seven days of their hearing loss recovered compared to 27% who presented thirty days or later. He noted that there is some self-selection bias whereby those that recover rapidly do not seek medical aid.
- Age – The average age for those recovering totally was 41.8 years. Those under 15 years and over 60 years had significantly poorer recovery rates.
- Vertigo – Patients with severe vertigo had significantly worse outcomes than patients with no symptoms of vertigo. 29% of patients with vertigo recovered compared to 55% with no vertigo.
- Audiogram – Patients with profound hearing loss had significantly decreased recovery rates compared to all other groups (22% with complete recovery).
Other series have shown that patients with midfrequency hearing loss, particularly when hearing at 4000kHz was worse than 8000kHz, have an excellent prognosis. 100% of patients in Laird’s series in 1983 recovered completely. The majority of studies confirm the findings that profound hearing loss is a poor prognostic sign indicating more severe injury.
Conclusion
Rapidly progressive or sudden hearing loss no matter what the cause is a medical condition which can be particularly devastating to patients and frustrating for the otolaryngologist to diagnose and treat. Despite extensive investigation, only minimal data has been generated in the past thirty years to improve our understanding of the etiology and appropriate treatment of this disease. Most authorities agree that all patients should undergo audiometry, and imaging with MRI for those patients with asymmetric hearing loss, however the etiology for the majority of patients will go undiagnosed. Treatment is more controversial. Steroids have been shown to significantly improve hearing recovery in patients with moderate to severe hearing loss and seem to be favored for the treatment of autoimmune and idiopathic forms of SSNHL. The remainder of proposed treatments for this disease are based, for the most part, on theory and will require further investigation to confirm or disprove their efficacy.
Development of future treatments for AIED will be dependent on the understanding of the exact immunologic events that occur in patients with this disease. Current ongoing investigations are looking at the role of helper T lymphocytes in experimental models of AIED. In particular, scientists are looking at the role of Th2 lymphocytes which appear to be capable of inducing a state of immunological unresponsiveness. By understanding the pathophysiological role of gene products of these cells in autoimmune hearing loss or, more importantly, by delineating their potential role in the maintenance of “tolerance” of self antigens, novel immunotherapeutic strategies may evolve.
BALANCE FUNCTION TESTING
Physiology of Balance
Humans use three basic mechanisms to obtain a sense of balance in daily life. The three mechanisms (visual, vestibular, and proprioceptive) interact to maintain posture and impart a conscious sense of orientation. There are measurable reflexes associated with these stimulus modalities. Reflexes generally serve to maintain stability in posture (e.g. by extending muscle groups in the direction of an anticipated fall), or in maintaining stability of the visual field. A defect in one of these systems, or incongruous inputs amongst the systems can be compensated for by reliance on the other two systems. However, such a defect decreases the patient’s overall ability to adjust to incongruous stimuli between the other two fields. Also, a defect can result in a serious subjective feeling of disequilibrium in the affected patient until compensation for the deficit occurs.
Visual system:
Visual inputs aid in the maintenance of an upright posture and in orientation. Conscious and unconscious correction of posture is possible through processing of visual inputs. The adjustment of posture and sensation of movement in response to visual stimuli can be seen by observing individuals’ responses to optokinetic stimuli (repeated movement of large objects in the subject’s visual field). Such stimuli (e.g. a train moving on the adjacent platform) impart a sense of acceleration to the individual and lead to reflexive postural adjustments (e.g. leaning in the direction of the moving train) to maintain balance. Visual reflex arcs also aid in maintaining the stability of the visual field. The saccade system focuses a visual target of interest onto the fovea through a fast movement of the eyes. The smooth pursuit system allows fixation of gaze onto a moving object with a frequency of less than 1.2 Hz. The optokinetic reflex is a result of multiple objects moving through a patient’s visual field (with the moving objects occupying about 80% of the patient’s visual field). The optokinetic reflex imparts a sense of motion to the patient. It presents as a jerk nystagmus with the slow component in the direction of the moving objects and the fast component back to the midline.
Proprioceptive system:
Proprioceptive inputs aid in static and dynamic postural control primarily through two reflex arcs. The first is the myototic reflex (deep tendon reflex), in which stretch on a muscle causes contraction of the muscle. The myototic reflex serves to maintain stability across a joint. The second proprioceptive reflex arc that aids in posture control is the functional stretch response, which utilizes multiple somatosensory inputs to provide for coordinated limb and trunk movements across joints, for instance to maintain the center of gravity over the support base in an individual who is bumped from behind. This reflex pathway has a higher latency than the myototic reflex, although both are mediated through spinal pathways. Both of these reflex arcs have lower latencies than visual-postural reflexes and vestibular-postural reflexes.
Vestibular system:
The vestibular system consists of two groups of specialized sensory receptors: the semicircular canal and otolithic organs. The semicircular canal detects angular acceleration of the head. The semicircular canal consists of a membranous semicircle with a widened area, the ampulla, at one end. The ampulla contains the crista ampullaris: specialized ciliated cells (several small cilia and one large eccentric kinocilium on each cell) jutting into the lumen of the ampulla. The cilia are embedded in a gelatinous structure called the cupula. The membranous semicircular canal contains endolymph (extracellular fluid with a high potassium concentration) that is the same specific gravity as the cupula. When angular acceleration of the head occurs in the plane of the semicircular canal, the endolymph’s momentum causes it to stay relatively stationary, displacing the cupula slightly. The cupula then displaces the cilia, causing a decrease in the firing rate of the associated vestibular nerve if the cilia bend away from their kinocilium and an increase in vestibular nerve firing if the cilia bend toward their kinocilium. There are 6 total semicircular canals (three in each inner ear): paired lateral, superior and posterior. The lateral semicircular canals have a plane that is elevated 30 degrees from the coronal. The posterior and superior semicircular canals are in planes that are approximately 90 degrees from each other and both are laterally askew from the sagital plane. The superior (a.k.a. anterior) canal on one side is on the same plane as the posterior canal on the other side, and so detects angular acceleration in the same plane. The ampulla and kinocilia in the crista ampullaris of the lateral semicircular canal are arranged in such a way that ampullopetal flow of endolymph causes increased firing of the vestibular nerve and ampullofugal flow decreases the firing of the vestibular nerve. The arrangement is opposite to this in the posterior and superior canal, with ampulopetal flow leading to inhibition of the associated vestibular nerve branch and ampulofugal flow leading to excitation. The end result is the same though: when head is turned towards the side of the semicircular canal (in its plane), that side’s vestibular nerve is excited and the opposite paired side’s nerve is inhibited. The range of response in excitation of a nerve is greater than the range of response in inhibition. Therefore, both vestibular nerves are generally required to sense acceleration without any detectable deficit.
The otolithic organs consist of a utricle, which is oriented the axial plane, and a saccule, which is oriented in the sagittal plane. These structures contain ciliated cells underneath a gelatinous layer and are bathed in endolymph. They also contain otoconia, which are calcium carbonate crystals of a higher specific gravity than endolymph. The otoconia are displaced in response to changes in head position with relation to the vertical. The otolithic organs also respond to linear acceleration. The ciliated cells can inhibit or excite the vestibular nerve, depending on the direction of their bend in relation to the kinocilium (away=inhibition, towards=excitation).
The vestibular system can affect posture via vestibulospinal pathways. These pathways, in conjunction with visual-postural and proprioceptive-postural pathways, serve to maintain the patient’s center of gravity over the base of support. For instance a quick head tilt to the right causes extension of right sided leg extenders to counteract a change in the perceived center of gravity. A perceived forward motion causes a sway forward to maintain the support base.
The vestibulo-ocular reflex is a system that maintains the stability of the visual field in response to acceleration of the head in a particular direction. The pathway is from vestibule to vestibular nuclei to the ocular motor nuclei, with modulation from cerebellar centers. The reflex results in movement of the eye so that the fovea can focus the same image during movement of the head. Thus the eye rotates (including tortional rotation) in an exactly opposing fashion to the head. When the eye’s rotational limit is exceeded, a saccade brings the eye back to the midline. For example, rotation of the head (nose) to the left would result in excitation of the left branch of the vestibular nerve that innervates the left semicircular canal and in inhibition of the right semicircular canal branch. This combination of excitation and inhibition is passed through the reflex arc and is translated into excitation of the ocular muscles to rotate the eye to the right in an exactly opposing fashion to the head rotation until no longer possible, at which point a saccade brings the eye back to the midline. The process then repeats itself until the angular acceleration ceases. The eye movement in this example produces a physiologic left beating nystagmus. Similarly, excitation of the vestibular nerve branch inervating the left superior semicircular canal would lead to a down and left torsional beating nystagmus. Alternatively, inhibition of the right posterior canal would result in the same thing. Complete ablation of the left vestibular nerve would result in nystagmus beating to the right because of the tonic input from the right lateral canal. Torsional nystagmus beating to the right would also be noted because of the superior and posterior canal on the right. Up-beating or downbeating nystagmus cannot be explained by a lesion in the periphery, and so, is almost always due to central etiologies. The otolithic sensory systems can influence eye tilt, for example, elevating the right eye, and depressing the left when the head is tilted toward the right shoulder.
The vestibular system is a very important system in the conscious sensation of acceleration. Peripheral or central damage to the vestibular system would lead to a severe sense of imbalance until compensation occurrs. Also, they would result in measurable alterations of the vestibulo-spinal and vestibulo-ocular reflexes until compensation occurs. Compensation in peripheral vestibular injury is via adjustment of the gain of vestibular reflexes in the cerebellum and modification of signal delivery to supratentorial centers.
Types of Balance Function Testing:
The Bedside Evaluation:
Most causes of dysequilibrium can be diagnosed by a complete history and several basic bedside evaluations of the three balance systems. A discussion of the causes of dysequilibrium is beyond the scope of this article. Important questions in the history are directed towards obtaining a concrete description of the patient’s symptoms. An important symptom suggesting vestibular pathology is the sensation of vertigo. Vertigo should be defined for the patient as a sensation of motion, or feeling that the world is moving when no motion is actually occurring. The duration of this symptom, aggravating factors (e.g. position), associated symptoms of hearing loss, tinnitus, and affect on daily life should all be probed. When vestibular pathology is suspected, an audiogram is important to check for the presence of concomitant auditory pathology. Syncope and the “light-headedness” that precede syncope are generally not associated with vestibular problems.
The bedside examination of balance function includes a thorough neuro-otologic examination. The exam can help to distinguish between peripheral and central causes of vertigo and can help to determine the cause of peripheral lesions. General physical examination should include a vascular exam. Blood pressures from both arms can be checked for the presence of subclavian steal syndrome. Supine and standing blood pressure can test for orthostatic hypotension. Auscultation for cervical bruits can identify possible posterior circulation abnormalities (which uncommonly cause vertigo during TIA’s of the posterior circulation).
Proprioceptive and vestibulospinal function can be tested with a group of gross balance tests. The Romberg test involves having the patient stand feet together with the eyes closed to see if the patient can maintain balance. It tests vestibular (primarily otolith organs) and proprioceptive balance pathways. The vestibulospinal pathway can be isolated by having the patient stand on a foam surface to minimize proprioceptive input. The past-pointing test is primarily (though not completely) a test of proprioception, and it involves having the patient repeatedly bring his finger to a remembered position with his eyes closed. Patients with vestibular pathology may point more to the side with the lesion. The Fukuda stepping test involves having the patient step in place with the eyes closed about 20 times. The patient may turn towards the side of the lesion. It is important to note though that right handed people often drift to the left somewhat with this test.
Motor and sensory examination further tests proprioception and cerebellar function. Cerebellar function is tested with rapid alternating movements and finger-to-nose tests. Proprioception can be tested directly by flexing or extending body parts (e.g. toes) and asking patients to describe their positions. Proprioception is also tested by testing deep tendon reflexes.
Vision should be tested by testing visual acuity. Acute change in visual acuity in one eye may lead to loss of depth perception and to a sense of disequilibrium. Ocular nerve palsy can also affect binocular vision. Saccades and smooth pursuit should be evaluated. The optokinetic reflex can be tested by rotating a drum with vertical stripes that covers 80% of the patient’s visual field and observing the nystagmus patern.
Static vestibular balance can be evaluated by an assessment of nystagmus. Nystagmus is a pattern of back-and-forth eye movement with a slow and fast phase. The directionality of nystagmus refers to its fast phase. Spontaneous nystagmus is the hallmark of static vestibular imbalance. Characteristics that distinguish central from peripheral imbalance include the effect of visual fixation (which suppresses nystagmus from peripheral, but not central lesions), the wave-form of the slow phase (peripheral lesions are steady and beat in one direction, while cenral lesions may not), and the axis of eye rotation (which usually involves directional and torsional components in peripheral nystagmus). Visual fixation induced suppression of nystagmus may make detection of nystagmus difficult. The Frenzel lenses remove this obstacle. Peripheral nystagmus is a jerk nystagmus with unidirectional constant-velocity slow phases; but velocity of the slow phase increases when looking the direction of the quick phase. Nystagmus of a central etiology may have a decaying-velocity slow phase or a pendular nystagmus. Otolith imbalance may lead to a skew deviation (vertical misalignment of the eyes) and a head tilt toward the side of the ablative lesion. Skew deviation may also be seen with lesions of the medial longitudinal fasciculus (MLF) or a trochlear nerve paresis.
Dynamic vestibular function can be tested in several ways. The patient’s head can be turned to one side, then the other while asking the patient to fixate on a stationary object. This tests the gain of vestibulo-ocular reflex. Patients with an ablative lesion on one side exhibit saccadic eye movements during a turn to that side to keep focused on the target because the gain of the vestibulo-ocular reflex for that motion is less than 1. This is a result of decreased stimulus from that side. Head shaking nystagmus occurs after shaking a the patient’s head with a frequency of 2 Hz for at least 10 seconds, with a brief post-shaking nystagmus beating away from the ablative lesion.
Provocative measures can be used at the bedside to evoke nystagmus in patients. Hyperventilation may induce nystagmus in patients by increasing the excitability of depressed vestibular nerves (e.g. those compressed by a vestibular schwannoma), thus negating the central compensation that has occurred over time. Positioning is useful in the diagnosis of benign paroxysmal positional vertigo, a disorder involving posterior canal irritation secondary to otoliths slipping into the posterior canal (canalolithiasis). The Dix-Hallpike maneuver involves moving a patient from a seated position with the head turned 45 degrees to one side to the head-hanging supine position. This is repeated for the other side. The maneuver moves the otoconial debris into an irritating position, causing, after a latency of 1 to 10 seconds, an upbeating and torsional nystagmus with the fast phase of the torsional component towards the affected ear in reference to the upper pole of the eye. The nystagmus fatigues after 20 to 45 seconds. The valsalva maneuver, either with a closed glottis, or an open glottis and closed nose and mouth increases intracranial pressure, or middle ear pressure, respectively. This may cause vertigo in patients with superior canal dehiscence, perilymph fistulas, or in people with cranio-cervical abnormalities (Arnold-Chiari malformation).
Laboratory Tests of Balance Function:
There is wide range of laboratory tests to evaluate the balance system Each test has its own advantages and drawbacks and must be considered in light of the patient’s history and physical exam findings. Laboratory balance function tests cannot provide a diagnosis, but can give insight into the pathophysiology of the patient suffering from a balance disorder. Vestibular function tests can provide important information confirming the side involved in a peripheral vestibular lesion. Balance function tests can provide a quantitative measure of the extent of a vestibular lesion, which is particularly useful in monitoring progression of a lesion (e.g. in ototoxicity). Balance function tests of posture can provide a measure of the patient’s ability to integrate sensations from several different modalities. Vestibular function tests may also help to decide which patients would benefit most from vestibular rehabilitation.
Electronystagmography (ENG), and Infrared Cameras:
The ENG is the centerpiece of most formalized balance function testing. The ENG detects quantitative changes in eye position by measuring the change in natural charge difference between the retina (-) and cornea (+) that occurs with eye movement. Electrodes are placed to measure vertical and horizontal movements. The advantages of this recording methodology include the low expense, minimal patient discomfort, and audiologist experience with the technique. The disadvantages include the susceptibility of the signal to changes in skin resistance, eye blink artifacts, and poor signal-to-noise ratio. Newer technologies exist for recording eye movement as well. Infrared imaging systems can be used to quantitatively monitor and record eye movements via the use of goggles that emit infrared light and contain infrared sensitive cameras. Such systems allow visualization of the patient’s eyes on a TV monitor and allow recording of tortional eye movements, which the ENG cannot. Visual stimuli can be presented directly via the goggles. In addition to recording of tortional nystagmus, other advantages of the infrared systems include elimination of artifact, elimination of the need for frequent recalibration, and more easy identification of disconjugate eye movements. The disadvantages of these systems include the need for bulky goggles and the expense of the system. Both ENG and infrared detection systems measure eye movements to assess the saccade system, the smooth pursuit system, the optokinetic reflex, and the vestibulo-ocular reflex.
Saccades are rapid eye movements designed to bring a peripheral visual target onto the fovea. Most systems use a computer generated model for the presentation of visual targets to the patient and then characterize saccades in terms of velocity, accuracy, and latency. Abnormalities in the saccade system often result from central pathology. Undershooting or overshooting saccades implicate pathology in the cerebellum. Slow saccades can result from a variety of central lesions (especially the pontine reticular formation) and from the use of certain medications. Long latencies in the initiation of saccades may result from neurodegenerative disorders or from lesions of the brainstem and cerebellum
Smooth pursuit testing involves tracking a visual target as it moves back and forth in the visual field. Inability to smoothly pursue a target results in frequent corrective saccades, and can result from a broad range of CNS pathology, especially in the cerebellum or brainstem.
Optokinetic nystagmus testing involves the stimulation of the almost the entire retina (and not just the fovea). Rotating stripes that fill 80% of the visual field are frequently used. The eyes follow a stripe and then make a quick saccade to catch the next stripe, resulting in the pattern of optokinetic nystagmus. Lesions responsible for abnormalities in the slow phase of the nystagmus are similar to those responsible for smooth pursuit system defects.
The vestibulo-ocular reflex is the pathway assessed by the rest of the ENG or infrared system. Spontaneous nystagmus is measured by removing visual fixation (dark visual field). Spontaneous nystagmus is considered abnormal if its peak slow phase exceeds 5 degrees per second. Gaze-evoked nystagmus is tested by having the patient focus on targets 30-40 degrees to the right, left, above, and below the center. Gaze evoked nystagmus that is of peripheral vestibular origin is typically unidirectional and beating toward the side of greater neural activity, has both torsional and horzontal components, and its amplitude increases when gaze is directed toward the direction of nystagmus. Gaze-evoked nystagmus of central etiology may be purely vertical or torsional, it does not suppress with fixation, and it may change direction with gaze. End-point, or eccentric nystagmus may be physiologic when it is not sustained. Symmetric gaze-evoked nystagmus may result from drugs such as phenobarbital, alcohol, diazepam, or phenytoin.
Position testing can be static or paroxysmal. Static testing involves placing the patient in various positions relative to gravity with removal of visual fixation and observing the vestibular response. The significance of abnormalities in this modality are debatable and must be correlated with other findings in the ENG. Paroxysmal testing is embodied by the Dix Hallpike maneuver. Occasionally, it is necessary to perform formal testing to obtain a quantitative measure of the severity of disease in BPPV (for example, if surgery is to be performed, although it is now rare to perform surgical ablation for BPPV).
Bithermal Caloric tests are used to evaluate the lateral semicircular canal. The patient is placed at an angle 30 degrees to the horizontal to place the lateral canal in a vertical position. The external auditory canals are irrigated with water 7 degrees Celsius above and below the body temperature. Convective effects on the endolymph of the lateral canal produce very slow fluid flow (.002 to .004 Hz). The induced nystagmus for the cold stimulus beats to the opposite side and that for the warm stimulus beats to the same side. A directional preponderance can be calculated with ENG, with a difference of greater than 30% between ears being considered abnormal. Unilateral weakness, or bilateral weakness with history of labyrinthine disease or after use of ototoxic drugs is indicative of peripheral lesions. Bilateral reduced or absent responses without history of labyrinthine disease may be suggestive of central disease.
Testing for the presence of a labyrinthine fistula or superior canal dehiscence can be accomplished with the use of a tympanometer and an ENG setup. In this test, the patient is first placed in an upright position and visual fixation is removed. Next, a probe from an immittance bridge is placed in the ear canal and a seal is obtained. Pressure is then varied from 0-200 mm H2 O and held for approximately 15 seconds. Pressure is then decreased to – 400 mm H2 O and held for 15 seconds. Patient is questioned for subjective symptoms. The presence of nystagmus or subjective symptoms is suggestive of a fistula.
The rotatory chair apparatus uses a rotating chair to test the semicircular canals at a higher (and more physiologic) frequency than with caloric testing. The test is an integration of responses from both vestibuli, unlike caloric testing. The patient is rotated in a chair at a velocity of from .01 to 1.28 Hz. The slow component of the physiologically induced nystagmus is analyzed in terms of phase, gain, and symmetry of eye movement. Symmetry is measured by comparing the peak slow-wave velocities between left and right rotations of the patient. In uncomplicated acute ablative vestibular lesions, the symmetry measure shows weakness on the affected side, though confounding factors such as compensation, labyrinthine irritation, and cerebellar lesions may render the symmetry test unreliable. Rotatory chair testing is generally more palatable to patients than caloric testing (especially pediatric patients). It is useful in monitoring changes in vestibular function over time, in monitoring compensation after acute injury, and in monitoring residual labyrinthine function in patients with no response during caloric testing.
Dynamic Posturography:
Dynamic posturography quantitatively measures the patient’s ability to maintain an upright posture in the face of varying somatosensory, visual, and vestibular inputs. Six conditions are tested. In condition 1, the patient maintains a stable position with eyes open. In condition 2, a stable position with eyes closed is maintained. In condition 3, the visual surround is shifted so as to give visual perception of motion. In condition 4, the floor is tilted in relation to the visual surround to provide vestibular, proprioceptive, and visual sensation of motion. In condition 5, the floor is tilted with the patient’s eyes closed, thus providing only vestibular and proprioceptive sensation of motion. In condition six, the visual surround is moved equivalently with the floor, again depriving visual sensation of motion. The patient’s sway from upright in response to these situations is graded on the scale of 0 (fall) to 100 (no sway). Force plates on which he or she stands detect the patient’s sway. Posturography is useful in determining the patient’s particular strategy in balancing and to see whether he or she prefers one or more of the balance modalities. It is also very useful in following patients in vestibular rehabilitation programs. Movement coordination tests involve a disruptive movement of the patient to see if he or she can regain balance. Like posturography, this is a multi-modality test.
The following chart adapted from Bailey’s Textbook of Otolaryngology is an excellent review of how patients with particular disorders perform on formal balance function testing.
EVALUATION AND MANAGEMENT OF BELL’S PALSY
Introduction
From its etiology and pathogenesis to its appropriate management, much has been written over the past century regarding Bell’s palsy, yet little has been accepted. Medical management with steroids and antivirals continues to be controversial. Surgical management has been even more unsettled with extremes of opinion continuing to range for those who believe surgical decompression is mismanagement to those who advocate early decompression for all patients with a complete facial paralysis (1). Thus, the clinician is often confused regarding the appropriate management of a patient presenting with an episode of acute facial paralysis due to Bell’s palsy.
The term Bell’s palsy has been used to describe a facial paralysis of acute onset and limited duration, the etiology of which was considered idiopathic in the past. The clinical presentation is typically defined by the rapid onset of the facial palsy, minimal associated symptoms, and spontaneous recovery. The diagnosis of Bell’s palsy is made after the exclusion of all other possibilities. Even with this strict criteria, Bell’s palsy remains the most common diagnosis given to patients with acute facial paralysis.
Fortunately, the etiology of acute facial paralysis due to Bell’s palsy is becoming clearer. Historically, there have been two prevailing theories: 1) vascular congestion with secondary ischemia to the nerve and viral polycranioneuropathy. McGovern postulated autonomic vascular instability with spasm of the nutrient arterioles. This vasospasm would lead to ischemia, nerve edema, and secondary compression within the fallopian canal. The mechanisms responsible for such insults included cold temperatures or psychosomatic causes.
An alternative theory by Antoni in 1919 proposed the term acute infectious polyneuritis cerebralis acusticofacialis. McCormick subsequently postulated the etiologic agent to be the herpes simplex virus in 1972. Recent studies by Murakami strongly suggest that herpes simplex virus type 1 (HSV-1) is active in cases of Bell’s palsy. In this study, endoneural fluid from 11 of 14 patients undergoing transmastoid decompression during the acute phase of the disease displayed DNA fragments of HSV-1 via the polymerase chain reaction. None from this surgical group displayed evidence of herpes zoster or Epstein Barr virus in the obtained samples. None of the affected controls undergoing surgery for temporal bone trauma or tumors displayed either HSV-1 or herpes zoster in their fluid samples either. Of the patients undergoing decompression for Ramsay Hunt syndrome, none exhibited DNA fragments of HSV-1 in their samples while all had herpes zoster. Indeed, Schirm and Mulkins stated that the Murakami study was so well controlled that “it provides conclusive evidence that reactivation of HSV genomes from the geniculate ganglia is the most important cause of Bell’s palsy.”
Even more compelling was the finding of HSV-1 DNA in a temporal bone section of a patient dying six days after developing Bell’s palsy (8). An animal model for Bell’s palsy has been developed in which animals inoculated with HSV-1 demonstrate transient facial paresis, thus further supporting the idea that Bell’s palsy is the result of a viral inflammatory response that induces edema within the facial nerve.
The natural history of Bell’s palsy has been examined in an article by Peiterson who evaluated the outcomes of 1011 patients with Bell’s palsy who were not medically or surgically treated. It was found that this condition occurs in every decade of life with a mean age between 40-44 years. It is less common before the age of 15 and after the age of 60 years. The incidence in men and women is similar. Approximately 6-9% develop recurrent Bell’s palsy. Facial paresis alone occurred in 31% of patients, while the remainder had unilateral complete paralysis. Of those experiencing only a paresis, over 95% recover without sequelae. Of the remaining 69% with complete paralysis, some return of facial function is evident within 3 weeks in 85% and an estimated 71% of patients achieve a House-Brackmann grade 1 and 13% a House-Brackmann grade 2. The remaining 16% in this complete paralysis group have a fair to poor recovery (House-Brackmann grades 3-5). This subset of patients is the controversial group of patients that may benefit the most from medical or surgical intervention.
Thus, of all patients with either a complete or partial facial paralysis, approximately 85% recover to normal within one year without treatment. It was noted that in patients that experienced delayed recovery over 3 months, all developed sequelae such as diminished function or contracture with associated movements. Return of at least some facial function was noted in all patients.
Many reports exist in the literature that attempt to document the outcomes in Bell’s palsy. Unfortunately, there was a lack of an accepted facial function recovery reporting system until the American Academy of Head and Neck Surgery adopted the House-Brackmann facial nerve grading scale in 1984.
Evaluation of Acute Facial Paralysis
A careful history of the patient’s illness narrows the scope of the differential diagnosis and reduces the number of subsequent radiographic and serologic studies that are necessary. Most palsies of the face are sudden in onset and frequently evolve over 2-3 weeks after onset. This evolution results in either a complete degeneration of nerve fibers or an incomplete degeneration with evidence of recovery as demonstrated by either clinical or electrophysiologic means. Any palsy progression over 3 weeks should be evaluated for a neoplasm. Evidence of trauma is usually obvious as is acute or chronic otitis media with or without cholesteatoma. Herpes zoster oticus (Ramsay-Hunt syndrome) is manifest by a facial paresis or paralysis with a vesicular eruption over a distribution of a cranial nerve. Sensorineural hearing loss and vertigo may also be present in up to 20% of cases. Recurrent facial palsy may be seen in cases of Bell’s or Melkersson-Rosenthal syndrome. Bilateral facial palsy is most commonly seen in Guillain-Barre syndrome, as a manifestation of Lyme disease, or with intracranial tumors or infections
The physical examination includes a complete head and neck examination with microscopic evaluation of the ear and a thorough cranial nerve examination. The face is examined and is assessed for paresis (an incomplete paralysis) or a complete paralysis.
Audiometry should be obtained in all cases to assess for possible involvement of the eighth cranial nerve in some disorders. Formulation of a treatment plan is dependent on the identification of the 15% of patients with Bell’s palsy who do not fully recover. Both imaging studies and electrophysiologic studies of the facial nerve have been used in this aim. The site of the lesion is best determined with either CT or MRI and prognosis is best determined with electrophysiologic testing. High resolution CT (HRCT) may be obtained in cases to examination the fallopian canal in temporal bone trauma, mastoiditis, or cholesteatomas. HRCT is unable to demonstrate subtle facial nerve inflammation typically seen in Bell’s palsy. Unfortunately, MRI assessment of the facial nerve has been disappointing as well. MRI may demonstrate enhancement of the facial nerve in cases of Bell’s palsy or Ramsay-Hunt syndrome, however no correlation with the site or degree of enhancement has been made. The primary role of MRI in cases of suspected Bell’s palsy is to exclude the possibility of other mass lesions that can lead to facial nerve paralysis. Topographic testing including the Schirmer test, stapedial reflex assessment, lectrogustometry, and salivary flow has become obsolete due to the inability to predict clinical outcome. If the diagnosis remains uncertain, serologic studies can be considered to evaluate for Lyme disease, autoimmune disorders, or other central nervous system diseases.
Anatomy
Knowledge of the anatomy of the facial nerve is important in understanding the pathophysiology and management of acute facial paralysis of Bell’s type. The intracranial segment of the facial nerve and the nervus intermedius exit the brainstem adjacent to the pons, cross the cerebellopontine angle, and enter the internal auditory canal. The meatal segment of the facial nerve and the nervus intermedius remain in the anterior-superior quadrant of the IAC and enter the fallopian canal at the meatal foramen, superior to the transverse crest and anterior to the vertical crest (Bill’s bar). Ge and Spector (14) have determined that the narrowest portion of the fallopian canal is located at the meatal foramen (mean diameter .68 mm). Thus, the size of the foramen coupled with a tight arachnoid band located at this segment contributes to the constriction of the facial nerve at this point in Bell’s palsy. The labyrinthine segment of the nerve, encased within the narrowest portion of the fallopian canal, courses 2-4 mm to the geniculate ganglion, where the nerve takes an acute turn at the external genu to enter the middle ear. The tympanic segment (horizontal segment) courses a total of 11 mm slightly above the cochleariform process and oval window and turns into the second turn (pyramidal turn) inferior to the lateral semicircular canal. The mastoid segment (vertical portion) then descends 13 mm to the stylomastoid foramen.
Electrophysiology
Bell’s palsy induces a wide range of facial movement dysfunction from mild paresis to total paralysis. The pathophysiology associated with this injury is suspected to be due to edema within the facial nerve induced by HSV reactivation. Several electrodiagnostic studies have been devised to evaluate the neural damage following acute facial nerve paralysis and can be used for prognostic purposes. These tests attempt to measure the amount of neural degeneration that has occurred distal to the sight of the injury by measuring the muscle response to an electrically evoked stimulus. These tests rely on the physiological premise of neural injuries as described by Seddon (13). Injuries that produce only a conduction block within the nerve and do not disrupt axonal continuity are termed neuropraxia. In these cases, the nerve does not sustain permanent damage and no Wallerian degeneration occurs. All electrophysiologic studies (NET, MST, and ENoG) will be within normal limits. EMG will fail to demonstrate voluntary motor action potentials, as these cannot be conducted past the blockade. With more severe injuries, axoplasmic disruption (axonotmesis) or neural tubule disruption (neurotmesis) occurs. Axonotmesis describes a state of Wallerian degeneration distal to the lesion characterized by the preservation of endoneural sheaths of the motor axons. Electrically, the NET, MST, and ENoG will indicate rapid and complete degeneration (if pure axonotmesis). The EMG will not demonstrate any voluntary motor units, and after 10-14 days, myogenic fibrillation potentials become evident. The axons will regenerate through the intact neural tubules, allowing complete return of motor function to the muscle fiber innervated by that nerve fiber. Neurotmesis describes the destruction of the axon and surrounding support cells. The lesion leads to Wallerian degeneration distal to the site of injury with the electrophysiologic tests being similar to that of axonotmesis. The outcome in this injury, however, is less predictable. The nerve fibers may be unable to regenerate successfully which might result in a misdirection of fibers resulting in synkinesis and incomplete facial function. Electrodiagnostic tests can be used to differentiate nerve fibers that have undergone Wallerian degeneration, but these tests are unable to differentiate axonotmesis from neurotmesis.
The currently popular tests used to establish prognosis in Bell’s palsy include the NET (nerve excitability test), MST (maximum stimulation test), ENoG (electroneurography), and EMG (electromyography). The NET, MST, and ENoG are most applicable in the evaluation of acute paralysis (during the degeneration phase). Hilger first described the NET in 1964. It compares the current thresholds required to elicit minimal muscle contraction on the normal side of the face to those of the paralyzed side. During degeneration, the NET will show increasing side-to-side threshold differences. A difference of 3.5 mA is considered significant and suggests more severe degeneration and a higher likelihood of a poor outcome. The maximum stimulation test is similar to the NET except that it uses maximum rather than minimum stimulation and responses are judged as a difference in facial movement. The MST will show greater degrees of facial weakness with worsening degeneration of the nerve. Unlike the NET and MST, the ENoG provides a quantitative analysis of the extent of degeneration without being dependent on observer qualification. It is the most accurate of all the diagnostic tests available and has proven predictive power. For this test, there is a recording of the summation potential (compound action potential) for the involved and uninvolved sides. The sides are compared with one another and the degree of degeneration within the nerve is directly proportional to the amplitudes of the measured summation potentials. Thus, with ongoing Wallerian degeneration, the ENoG will demonstrate lower percentages of intact motor axons able to propagate a summation potential.
Nerve degeneration in Bell’s palsy typically occurs over a three-week period and the NET, MST, and ENoG will provide the most accurate information. Within the first three days after the onset of complete paralysis, the results of NET, MST, and ENoG will be inaccurate, as Wallerian degeneration has not yet occurred. The results during this time will be near normal. Because of this limitation, the prognosis cannot be established reliably until the fourth or fifth day when Wallerian degeneration of damaged nerve fibers is likely ongoing. Comparing ENoG results during the course of the degeneration is important for prognosis. Esslen (14) found that if there is greater than a 90% degeneration of the amplitude of the ENoG waveform on the affected side, the prognosis worsens. This study established that those patients with 90-97% degeneration, 30% recovered fully; of those patients with 98-99% degeneration, 14% recovered fully; and those with 100% degeneration, none recovered fully.
To further quantify patient outcomes using ENoG, Fisch found that all patients having less than 90% maximal degeneration of facial nerve fibers within three weeks of onset of the palsy reach a satisfactory return of facial motion without any form of treatment (comparable to a House-Brackmann stage 1-2). On the other hand, 50% of the patients with a 95-100% maximal degeneration within two weeks of onset of the palsy have a permanent unsatisfactory recovery of function (House-Brackmann grades 3,4, or 5). He also found that if the nerve did reach 90% degeneration, there was a high likelihood of degenerating further to the critical 95% degeneration point. Thus, in these patients, the likelihood of normal to near normal recovery remains 50%.
EMG testing in the acute phase is primarily a complementary test as it is unable to distinguish a totally neuropraxic injury from a completely degenerated nerve from a regenerating nerve in the acute phase. For example, it is not uncommon for an ENoG test to record no response with early recovery in Bell’s palsy. This is because regenerating nerve fibers fire at different rates, which does not allow a complete summation potential to be recorded on ENoG. In these cases, EMG is essential to assess if motor units are observed with voluntary contraction. EMG is also essential in the long-term evaluation of facial nerve paralysis. The presence of myogenic fibrillation potentials and the absence of voluntary motor units denotes complete degeneration while the coexistence of both defibrillation potentials and motor units indicates an incomplete lesion and the appearance polyphasic motor units signifies a regenerating nerve.
Medical Management of Bell’s Palsy
It is difficult to prove that medical management influences the course of recovery in patients with Bell’s palsy. The most widely evaluated intervention is steroid therapy. In 1987, Stankiewitz (16) reviewed the literature on steroid use in Bell’s palsy and found no irrefutable study showing the efficacy of steroid therapy. In 1993, however, Austin (17) conducted a randomized, double blind, placebo controlled study that revealed an improvement in grade of recovery with the use of prednisone. All patients treated with prednisone were in the good outcome group of grades 1-2 on the House-Brackmann scale. However, 17% of patients not treated with prednisone fell into grade 3 recovery. These results were statistically significant. A trend towards the prevention of denervation was noted but did not reach clinical significance. The authors felt that prednisone treatment was most likely to be beneficial in patients with more severe paralysis at initial presentation.
Given the likely association of Bell’s palsy with HSV reactivation, Adour (18) conducted a double blind study comparing prednisone and placebo and prednisone and acyclovir. The drugs were initiated within three days of onset of the paralysis. Only 20% progressed to complete paralysis clinically within 14 days of onset and these were evenly distributed between the groups. The acyclovir treated patients demonstrated less degrees of facial weakness (and presumably less degeneration) on MST testing and a lower incidence of unsatisfactory recovery (House-Brackmann grade 3-5).
Given these results, most authors recommend a medical regime consisting of both steroids and antivirals, with most recommending initiation within three days of onset. Protection from corneal irritation is critical with hourly saline eye drops and nightly ophthalmic ointment in the eyes. Regular, scheduled assessment of facial function is important so that appropriate prognostic electrophysiologic tests can be administered in the event of complete paralysis.
Surgical Management of Bell’s Palsy
There is spirited debate among clinicians concerning the appropriate surgical management of the Bell’s palsy patient since it was first reported in 1932 by Balance and Duel. Fowler commented in 1939 that there was great controversy regarding the indications for such a procedure and that the procedure be done only by experts. Facial nerve surgery was greatly influenced in the 1960’s with the advances in electrodiagnosis. During this decade, the site of the lesion was felt to be the tympanic segment. Thus, most cases of decompression were focused at that site. In the 1970’s, McNeill reviewed his patients treated with postauricular decompression from the geniculate to the stylomastoid foramen and found no appreciable benefit. All of these patients were operated on after two weeks. In 1972, Fisch and Esslen published revolutionary data in the surgical management of Bell’s palsy. They reported 12 patients who had undergone total facial nerve decompression via a middle cranial fossa and transmastoid approach. Using intraoperative electrical stimulation in three patients, they found a conduction block in the region proximal to the geniculate ganglion (in the area of the meatal foramen and labyrinthine segment), consistent with their gross anatomic observations. Fisch (15) later went on to conclude that decompression of the facial nerve in patients with 90% reduction of the compound action potential should be done within two weeks for maximum benefit. He found that there was a statistically significant benefit to decompressing the meatal foramen in this selected group of patients. May initially reported that transmastoid decompression to the labyrinthine segment was beneficial in selected patients (decreased salivary flow, decreased Shirmer test, and MST reduced to 25% of normal). In a subsequent publication, he recanted these ideas by finding that no patients benefited from transmastoid decompression performed within 14 days of onset. Thus, two schools of thought developed in the 1980’s and 1990’s; those who adopted the idea of early intervention debated the ideal approach while those who opposed surgical management were quite emphatic.
In order to help define which patients would benefit from surgical decompression and to corroborate the original findings of Fisch (15), Rubenstein and Gantz undertook a retrospective study in 1999 to assess whether patients with severe degeneration (greater than 90% on ENoG) would benefit from decompression of the facial nerve. In this study, the meatal foramen, labyrinthine segment, geniculate ganglion, and tympanic section were decompressed through a middle cranial fossa approach. This route was chosen because of strong evidence that the conductive block occurs in and around the meatal foramen in 94% of patients (using intraoperative EMG) and that electrical impulse conduction improves after decompression of that area. If the conduction block was not identified during this approach using intraoperative EMG, a transmastoid decompression was added. They reported in a multi-institutional review that 92% of patients who exhibited greater than 90% degeneration on ENoG within 14 days recovered to a House-Brackmann grade 1 or 2. In patients who were treated only with steroids, only 45% recovered to a House-Brackmann grade 1-2.
They concluded that facial nerve decompression for Bell’s palsy is useful in a selected group of patients—those with greater than 90% compound action potential reduction within 14 days on ENoG and lack of volitional activity on EMG.
Conclusion
It is clear that the management of acute facial paralysis is evolving. Many unresolved issues remain. Hopefully, through diligent scientific inquiry, superior diagnostic and therapeutic methods will be discovered for the treatment of Bell’s palsy.
BELL’S PALSY
Bell’s palsy is the most common diagnosis given to patients with acute facial palsy. Despite substantial effort to study its disease process, the management of Bell’s palsy remains controversial. This manuscript serves not as a treatment protocol for Bell’s palsy, but a review of literature on the subject.
Bell’s palsy was named after Sir Charles Bell (1774-1842). His most notable contribution is arguably his description of the facial nerve as the “respiratory nerve of the face” that controls our facial expression. The motor nucleus of the facial nerve lies deep within the reticular formation of the pons where it receives input from the precentral gyrus of the motor cortex, which innervates the ipsilateral and contralateral forehead. The cerebral cortical tracts also innervate the contralateral portion of the remaining face. This accounts for the sparing of the forehead motion in supranuclear lesions of the facial nerve.
The parasympathetic secretory fibers of the nervous intermedius arise from the superior salivatory nucleus. These preganglionic fibers travel to the submandibular ganglion via the chorda tympani nerve to innervate the submandibular and sublingual glands, and to the sphenopalatine ganglion via the greater superficial petrosal nerve to innervate the lacrimal, nasal, and palatine glands. The secretory fibers of the lesser superficial petrosal nerve traverse the tympanic plexus, synapse in the otic ganglion, and travel via the auriculotemporal nerve to innervate the parotid gland. Taste fibers from the anterior 2/3 of the tongue reach the geniculate ganglion via the chorda tympani nerve and from there travel to the nucleus of the tractus solitarius.
The facial nerve and nervus intermedius exit the brain stem at the pontomedullary junction and travel laterally 12 – 14 mm with cranial nerve VIII to enter the internal acoustic meatus. The meatal segment of the nerve then travels 8 – 10 mm within the internal auditory canal (anterosuperior quadrant) to the meatal foramen where the canal narrows from 1.2 mm in diameter to 0.68 mm in diameter (the narrowest part of the canal). The labyrinthine segment then runs 2 – 4 mm to the geniculate ganglion. Here the greater superficial petrosal nerve exits to carry parasympathetic secretomotor fibers to the lacrimal gland. Just distal to this branch, the lesser superficial petrosal nerve exits to supply parasympathetic secretomotor fibers to the parotid. The tympanic segment begins just distal to the geniculate ganglion where the nerve turns 40 to 80 degrees (first genu) and runs posteroinferiorly 11 mm across the tympanic cavity to the second genu. A branch leaves the segment near the pyramidal eminence to supply the stapedius muscle. The nerve then turns about 90 degrees at the second genu inferiorly where the mastoid segment travels for 12 – 14 mm inferiorly in the anterior mastoid to exit the stylomastoid foramen. The terminal branch of the nervus intermedius, the chorda tympani, leaves the mastoid segment 5 mm proximal to the foramen and travels lateral to the incus, medial to the malleus to exit at the petrotympanic fissure. The extratympanic segment is composed entirely of motor fibers and enters the parotid gland after giving off the posterior auricular branch and a branch to the posterior belly of the digastric muscle. The pes anserus forms 20 mm from the stylomastoid foramen and further divides the nerve into the upper (temporal, and zygomatic) and lower (buccal, mandibular, and cervical) branches.
Although Bell’s palsy is a diagnosis of exclusion, it is the most common diagnosis given for acute facial palsy (> 60%). It causes peripheral facial neuropathy that tends to be unilateral and has a rapid onset. Its incidence is about 30 per 100,000. There is an equal male to female ration and a 3.3 times greater incidence in pregnant females. The left and right sides of the face are equally involved, and less than 1% of cases are bilateral. The recurrence rate is about 10% and can be ipsilateral or bilateral. Patients with diabetes have 4 – 5 times more risk of developing the disease. A family history is positive in about 10% of patients with Bell’s palsy.
In 1982, Peitersen et al. published an article on the natural history of Bell’s palsy based on more than a thousand Danish patients. This study is unique in that it evaluates the spontaneous course of the disease without medical or surgical intervention. He found that Bell’s palsy occurred in every decade of life, with a mean age of between 40 and 44 years. It was less common before the age of 15 and after the age of 60 years. Total unilateral facial paralysis occurred in 71%. Common symptoms included reduced stapedial reflex, postauricular pain, dysgeusia, reduced lacrimation, and phonophobia. The prognosis for Bell’s palsy is generally good with 85 % of patients recovering completely within one month. The remaining 15% progress to complete degeneration and will not usually show signs of recovery for three to six months. The longer the time needed for recovery, the greater the probability of sequelae. Patients with incomplete paralysis will recover with no sequelae 95% of the time. Based on this study, poor outcome of Bell’s palsy is associated with advanced age, late return of muscular function or beginning of remission, complete palsy, abnormal taste, stapedial reflex, and lacrimation.
Although Bell’s palsy has been described as idiopathic facial paralysis, there are increasing evidence suggesting a viral etiology. A study by Murakami et al. strongly suggests that herpes simplex virus type 1 (HSV-1) is active in idiopathic facial paralysis. DNA fragments of HSV-1 were exclusively found in the perineural fluid of Bell’s palsy patients who underwent surgical decompression. An Iowa group has also identified HSV-1 DNA in a temporal bone section of a patient dying 6 days after developing Bell’s palsy. These two independent pieces of evidence strongly support the concept that the facial paralysis associated with Bell’s palsy is the result of a viral inflammatory response that induces edema within the facial nerve.
The first step in evaluating any patient who presents with facial nerve paralysis involves taking a careful and thorough history. It is important to determine the onset of the paralysis (sudden vs delayed), the duration, and the rate of progression. It is especially important to determine whether the paralysis is complete verses incomplete. Patients should be questioned regarding previous episodes, family history, associated symptoms (hearing loss, otorrhea, otalgia, vertigo, headaches, blurred vision, parasthesias), associated medical illnesses (diabetes, pregnancy, autoimmune disorders, cancer), history of trauma (recent or remote), and previous surgery (otologic, rhytidectomy, parotidectomy).
A complete head and neck examination must be performed, including microscopic examination of the ears, careful palpation of the parotid glands and neck, ophthalmologic examination (r/o papilledema), auscultation of the neck ( r/o carotid bruits), and a thorough neurological examination. It is important to assess the degree of voluntary movement present in order to document the grade of facial paralysis as described in the House classification system:
Any patient presenting with facial paralysis should undergo formal audiological testing, including pure tone, air and bone conduction, speech discrimination, reflexes, and tympanometry. If asymmetry is found on the audiogram, an ABR and/or MRI should be obtained.
The most likely site of lesion in Bell’s palsy is the meatal foramen (junction of the internal auditory canal portion of the nerve and the labyrinthine segment of the nerve), which is considered to be the narrowest portion of the fallopian canal. MRI with gadolinium will usually show enhancement of the labyrinthine portion of the nerve. As the edema within the nerve increases, axonal flow and circulation are inhibited resulting in varying degrees of nerve injury (first, second, and third degree). Patients who are most severely affected develop a high level of third degree injury that can result in the loss of endoneural tubules and misdirected axonal regeneration. Histological studies from patients with Bell’s palsy who died of nonrelated causes reveal diffuse demyelination of the facial nerve with lymphocytic infiltrates.
The principle behind topognostic testing is that lesions distal to the site of a particular branch of the facial nerve will spare the function of that branch. Moving distally from the brainstem, these tests include: the schirmer test for lacrimation (GSPN), the stapedial reflex test (stapedial branch), taste testing (chorda tympani nerve), salivary flow rates and pH (chorda tympani).
Although these tests are of historical interest, they have not been found to be of much use clinically for determining the site of the lesion in facial paralysis or for predicting the outcome. Marked discrepancies are often seen. For example, patients may exhibit a marked decrease in lacrimation with a normal stapedial reflex and intact taste, or they may have absent lacrimation and an absent stapedial reflex with normal salivation. These discrepancies are easily explained in Bell’s palsy, where there can be multiple sites of inflammation and demyelinization from the brainstem to the peripheral branches of the nerve.
Electrical tests are useful for patients with complete paralysis for determining prognosis for return of facial function and the endpoint of degeneration by serial testing. They are most useful when considering decompression surgery and are of no value in patients with incomplete paralysis.
The nerve excitability test (NET), maximal stimulation test (MST), and electroneuronography (ENoG) are most useful in the degenerative phase. These tests will give normal results during the first 72 hours after injury due to the stimulating and recording electrodes both being distal to the site of the injury. After 3 – 4 days, the nerve degeneration reaches the site of stimulation and useful results will be obtained. These tests can only be used for unilateral paralysis because all three involve comparison to the contralateral side that must be normal for valid results.
ENoG and electromyography (EMG) are employed more often than NET and MST as the latter two modalities rely on subjective evaluation of muscular response, whereas the former two quantitatively measure compound muscle action potential.
For ENoG, the facial nerve is stimulated with an impulse transcutaneously at the stylomastoid foramen using bipolar electrodes. The muscular response is then recorded using bipolar electrodes placed near the nasolabial groove. The amplitude of the evoked compound action potential is considered proportional to the number of intact axons. The two sides are then compared with the response on the paralyzed side of the face expressed as a percentage of the response on the normal side of the face. A reduction in amplitude on the involved side to 10% or less of the normal side indicates a poor prognosis for spontaneous recovery. Fisch et al. notes that a maximal reduction of less than 90% within 3 weeks of onset gives an expected spontaneous rate of recovery of 80 – 100%. Disadvantages of ENoG include discomfort, cost, and test-retest variability owing to positioning of the electrodes and excitation of the muscles of mastication (V).
EMG is of limited value early in the evaluation of facial paralysis because fibrillation potentials indicating axonal degeneration do not appear until 10 to 14 days post onset. However, EMG becomes important for assessing reinnervation potential of the muscle two weeks after onset. By using needle electrodes placed transcutaneously into the muscles of facial expression, muscle action potentials generated by voluntary activity can be recorded. Electrical silence can indicate normal muscle in a resting state, severe muscle wasting and fibrosis or acute facial paralysis in the early stages. During normal voluntary contraction organized diphasic or triphasic potentials are seen. Fibrillation potentials indicate degeneration of the neural supply to the muscle in question. Polyphasic potentials indicate reinnervation. These are important because they usually appear 6 – 12 weeks before clinical return of function. It is generally obtained if ENoG displays more than 95% degeneration.
Sunderland’s classification describes five degrees of nerve injury. The first degree (neuropraxia) involves a localized conduction block in the nerve with the nerve fibers responding to electrical stimuli proximal and distal to the lesion, but not across the injured segment. Axonal continuity is preserved, wallerian degeneration does not occur, and recovery is usually complete.
The second degree of nerve injury is called axonotmesis. This refers to disruption of the axon into proximal and distal portions with interrupted axoplasmic flow and Wallerian degeneration. The third, fourth, and fifth degree of nerve injury are grouped together as neurotmesis, and subdivided depending on the integrity of perineurium and epineurium. Wallerian degeneration occurs at the faster rate than axonotmesis and prognosis is the poorest. The rate of nerve degeneration in Bell’s palsy falls in between axonotmesis and neurotmesis. However, there is no electrical test to-date that can quantitatively differentiate the two subclasses of injury.
Treatment options of Bell’s palsy range from observation, medical treatment, surgical decompression, to facial rehabilitation. The efficacies of oral prednisone and anti-viral agents have been studied extensively, yet there is no consensus among experts on ideal regimen and dosage. Cochrane review summarizes four trials (179 patients) that compare steroid to placebo, saline, and vitamin solutions. Its results show no significant benefit of giving steroid to Bell’s palsy patients. A similar conclusion is reached by Turk Boru’s randomized controlled trial (56 patients). Ramsey, however, points out in his meta-analysis that the rate of complete recovery (Grade VI I) is 17% higher in the steroid group, provided the total prednisone dose is at least 400 mg and that the treatment is initiated within a week of onset of paralysis (3 trials, 230 patients). The addition of anti-viral agent such as acyclovir is advocated by Adour, whose double-blind randomized controlled trial shows that the combined therapy of prednisone and acyclovir results in better return of muscle function and prevention of partial nerve degeneration. This result is challenged by another prospective trial by Kawaguchi who illustrates that valacyclovir plus prednisone have no advantage over prednisone alone. Regardless of medical regimen, early administration within 3 days of onset appear to have superior effect on outcome, as demonstrated in a study by Hato et al.
Eye care is of utmost importance in facial nerve paralysis due to the risk of exposure keratitis. Artificial tears and lacrilube ointment should be prescribed. Taping of the eye lids during sleep may be helpful as well as the use of a moisture chamber. Patients should avoid contact lens, fans and dust, and should have eye protection when outside in the wind. Gold weight implant to the upper eyelid should be considered in patients with long-standing facial paralysis.
Surgical decompression for Bell’s palsy has evolved in many ways throughout the years. The emphasis has shifted from focusing on the stylomastoid foramen in the 1930s to decompressing the meatal foramen nowadays, the narrowest portion of the facial canal. Proponents of surgical decompression such as Gantz believe in early decompression within 2 weeks of onset of paralysis if ENoG demonstrates more than 90% degeneration and EMG shows no voluntary muscle potential. Those who are against decompression argue that the Bell’s palsy is a result of viral demyelination and that increased intracanal pressure may not be the primary cause of Bell’s palsy. Although the issue of surgical decompression remains highly controversial, factors such as age, medical comorbidities, endpoint and rate of progression of ENoG, days from onset of paralysis, and when muscle function begins to return likely influence the outcome and allow us to predict the success of surgery.