Otology 2
CHOLESTEATOMA
Anatomy
Cholesteatoma growth patterns are predictable in that they are channeled along characteristic pathways by ligaments, folds, and ossicles. The most common locations from which cholesteatoma arise are the posterior epitympanum, the posterior mesotympanum and the anterior epitympanum. A basic knowledge of the anatomy of this area provides a foundation for understanding the disease progression and concepts for surgical management.
The middle ear can be divided into three compartments: the mesotympanum, hypotympanum, and epitympanum. The boundary that defines these areas is the external auditory canal. The epitympanum is superior and medial to the superior aspect of the external auditory canal. The hypotympanum is inferior and medial to the inferior aspect of the external auditory canal. The mesotympanum is medial to the external auditory canal with its inferior and superior boundaries defined by the inferior and superior aspect of the external auditory canal respectively.
The mesotympanum contains the stapes, long process of the incus, handle of the malleus and the oval and round windows. The eustachian tube exits from the anterior aspect of the mesotympanum. Two recesses extend posteriorly from the mesotympanum that are often not visible directly- the facial recess and sinus tympani. The facial recess and sinus tympani, are the most common location for cholesteatoma persistence after chronic ear surgery. The facial recess is lateral to the facial nerve, bounded by the fossa incudis superiorly and the chorda tympani nerve laterally. This recess may be directly accessed through a posterior approach via the mastoid (posterior tympanotomy or facial recess approach).The sinus tympani lies between the facial nerve and the medial wall of the mesotympanum and is very difficult to access surgically.
The epitympanum lies above the level of the short process of the malleus, containing the head of the malleus, body of the incus, and their associated ligaments and mucosal folds. The annular ligament sends off fibrous bands from the anterior and posterior tympanic spines that meet at the neck of the malleus. The dehiscent area in the tympanic bone, known as the notch of Rivinus, lies above these bands. The dense fibers that form the middle layer of the pars tensa do not extend to the pars flaccida. The lack of this structural support predisposes Shrapnell’s membrane to retraction when negative middle ear pressure is present secondary to Eustachian tube dysfunction.
Cholesteatomas of the epitympanum start in Prussack’s space between the pars flaccida and neck of the malleus with the upper boundary being the lateral mallear fold. The most common spread patterns of cholesteatomas from Prussack’s space are through the posterior epitympanum, posterior mesotympanum and anterior epitympanum. The most common spread pattern of the three is the posterior epitympanic route where the cholesteatoma spreads to the superior incudal space lateral to the body of the incus potentially gaining access to the mastoid through the aditus ad antrum. The second most common is the inferior route, thought the posterior pouch of von Troeltsch. This pouch lies between the tympanic membrane and the posterior mallear fold. Spread via this route allows cholesteatoma to gain access to the regions of the stapes, round window, sinus tympani and facial recess. Anterior epitympanic cholesteatomas form anterior to the malleus head. They may be easily overlooked during tympanomastoidectomy if the area is not explored. Facial nerve dysfunction may occur with these lesions, which can also gain access to the supratubal recess of the middle ear via the anterior pouch of von Troeltsch.
As previously described, the hypotympanum is the portion of the middle ear that lies inferior and medial to the floor of the bony ear canal. It is an irregular bony groove that is seldom involved by cholesteatoma. Occasionally, the jugular bulb may be dehiscent in this area.
Prevention of Cholesteatoma Formation
When a patient evaluated in clinic is noted to have a retraction pocket, the otolaryngologist must recognize that this manifestation is due to Eustachian tube dysfunction and that the condition precedes the development of acquired cholesteatoma. As a result, a long-term tympanostomy tube should be placed to resolve the negative middle ear pressure. This intervention may allow the tympanic membrane to revert to a neutral position. If the retraction pocket is adherent to the ossicles or folds or if it has been present for an extended period of time, the retraction pocket will persist. If the retraction pocket persists, surgical exploration may be indicated.
Patient Evaluation
As always, the initial patient evaluation should include a thorough history. A detailed otologic history should be obtained in order to elicit the early symptoms of cholesteatoma including hearing loss, otorrhea, otalgia, nasal obstruction, tinnitus and vertigo. A previous history of middle ear disease, such as chronic otitis media and/or tympanic membrane perforation may be revealed. Progressive unilateral hearing loss with a chronic foul smelling otorrhea should raise suspicion.
In addition to a thorough head and neck examination, the otologic examination should be meticulous and complete. Otomicroscopy is of the utmost importance in evaluating the presence of cholesteatoma and extent of disease. The ear should be thoroughly cleaned of otorrhea and debris with cotton-tipped applicators or suction. A retraction pocket may be seen, often in the attic and posterosuperior quadrant of the tympanic membrane. Accumulation of squamous debris may occur within the pocket. Granulation tissue may arise from the diseased infected bone of the scutum or posterior bony wall. When extensive, a polyp may protrude through an attic defect. Extreme caution should be used with polyp removal as it may be adherent to important underlying structures such as the ossicles or facial nerve. Pneumatic otoscopy should be performed in every patient with a cholesteatoma. A positive fistula (pneumatic otoscopy will result in nystagmus and vertigo) response suggests erosion of the semicircular canals or cochlea. Cultures should be obtained with wet, infected ears. Topical and/or oral antibiotics should be administered in these cases.
Pure tone audiometry with air and bone conduction, speech reception thresholds, and word recognition usually reveal a conductive hearing loss in the affected ear. The degree of conductive loss will vary considerably depending on the extent of disease. A moderate conductive deficit in excess of 40 dB indicates ossicular discontinuity, usually from erosion of the long process of the incus or capitulum of the stapes. A mild conductive deafness may be present with extensive disease if the cholesteatoma sac transmits sound directly to the stapes or footplate. Audiometry results should always be correlated with the 512Hz tuning fork exam. Tympanometry results will vary and may suggest decreased compliance or perforation of the tympanic membrane.
Preoperative imaging with computed tomographies (CTs) of the temporal bones (2mm -section without contrast in axial and coronal planes) allows for evaluation of anatomy, which may reveal evidence of the extent of the disease as well as screen for asymptomatic complications. Although a temporal bone CT is not essential for preoperative evaluation, they should be obtained for revision cases due to altered landmarks from previous surgery, for patients with complications of chronic suppurative otitis media, suspected congenital abnormalities, or cases of cholesteatoma in which sensorineural hearing loss, vestibular symptoms, or other evidence of complications exist.
Preoperative counseling is an absolute necessity prior to surgery. The primary objective of surgery is a safe dry ear which is accomplished by treating all supervening complications, removing diseased bone, mucosa, granulation polyps, and cholesteatoma while preserving as much normal anatomy as possible. Improvement of hearing is a secondary goal. Possible adverse outcomes must be discussed including facial paralysis, vertigo, further hearing loss, and tinnitus. The patient should understand that long-term follow-up will be necessary and that they may need additional surgeries.
Surgical Management
Cholesteatoma is treated surgically with a primary goal of total eradication of cholesteatoma to obtain a safe and dry ear. The second objective is restoration or maintaining the functional capacity of hearing. The third objective is to maintain a normal anatomic appearance of the ear if possible. The surgical procedure to be used should be designed for each individual case according to the extent of disease. More extensive disease will usually dictate a more aggressive surgical approach.
Canal-Wall-Down (CWD) Procedure
Prior to the advent of the tympanoplasty, all cholesteatoma surgery was performed using a CWD approach. This procedure involves taking down the posterior canal wall to the level of the vertical facial nerve and exteriorizing the mastoid into the external ear canal. The epitympanum is obliterated with removal of the scutum, head of the malleus and incus. A classic CWD operation is the modified radical mastoidectomy in which the middle ear space is preserved. The radical mastoidectomy is a CWD operation in which the middle ear space is eliminated and the eustachian tube is plugged. Meatoplasty should be large enough to allow good aeration of the mastoid cavity and permit easy visualization to facilitate postoperative care and self cleaning. The indications for this as an initial approach are cholesteatoma in an only hearing ear, significant erosion of the posterior bony canal wall, history of vertigo suggesting a labyrinthine fistula, recurrent cholesteatoma after canal-wall-up surgery, poor eustachian tube function, and a sclerotic mastoid with limited access to the epitympanum.
The advantages of the CWD procedure are that residual disease is easily detected, recurrent disease is rare, and the facial recess is exteriorized. The major disadvantage of this procedure is the open cavity and that mastoid bowl maintenance can be a lifelong problem. Healing takes longer in open cavities and the middle ear is shallow and difficult to reconstruct. Dry ear precautions are essential.
Canal-Wall-Up (CWU) Procedure
The CWU procedure was developed to avoid the problems and maintenance necessary when CWD procedures are performed. CWU consists of preservation of the posterior bony external auditory canal wall during simple mastoidectomy with or without a posterior tympanotomy. A staged procedure is often necessary with a scheduled second look operation at 6 to 18 months for removal of residual cholesteatoma and ossicular chain reconstruction if necessary. The procedure should be adapted to the extent of disease as well as the skill of the otologist. This approach may be indicated in patients with a large pneumatized mastoid and a well aerated middle ear space, suggesting good eustachian tube function. CWU procedures are contraindicated in only hearing ears or in patients with a labyrinthine fistula, long-standing ear disease, or poor eustachian tube function.
The advantages of CWU compared with CWD mastoidectomies are more rapid healing time, easier long-term care, no dry ear precautions, and hearing aids are easier to fit and wear if they are needed. The disadvantages associated with this procedure are the difficulty of technique leading to longer operative time, residual disease is more difficult to detect, retraction pockets leading to recurrent disease are possible, and staged operations are often necessary.
Transcanal Anterior Atticotomy
A transcanal anterior atticotomy is indicated for limited cholesteatoma involving the middle ear, ossicular chain, and epitympanum. If the extent of the cholesteatoma is unknown, this approach can be combined with a CWU mastoidectomy or extended to a CWD procedure. The atticotomy involves elevation of a tympanomeatal flap via an endaural incision with removal of the scutum to the limits of the cholesteatoma. After removal of the disease, the aditus is obliterated with muscle, fascia, cartilage or bone prior to reconstruction of the middle ear space. Some advocate reconstruction of the lateral attic wall with bone or cartilage, however, this may lead to retraction disease and possible recurrence in patients with poor eustachian tube function.
Bondy Modified Radical Mastoidectomy
Although rarely used today, the Bondy procedure is a useful for specific types of cholesteatoma. It is indicated for attic and mastoid cholesteatoma that does not involve the middle ear space and is lateral to the ossicles. Preferably, the mastoid should be poorly developed for creation of a small cavity. The eustachian tube function should be adequate, with an intact pars tensa and aerated middle ear space. The Bondy procedure is performed like the modern modified radical mastoidectomy with the exception that the middle ear space is not entered.
Complications
The expansion of cholesteatomas combined with the propensity of infection result in numerous complications that include ossicular chain destruction, exposure of the membranous labyrinth, exposure of the facial nerve and dura, and infection of the mastoid and intracranial spaces.
Conductive hearing loss is a common complication of cholesteatoma as ossicular chain erosion occurs in 30% of cases. Erosion of the lenticular process and or stapes superstructure may produce a conductive hearing loss as high as 50dB. However, hearing loss may vary with the development of a natural myringostapediopexy or transmission of sound through a cholesteatoma sac to the stapes or footplate. This results in less of a conductive hearing loss. The ossicular chain should always be assumed to be intact. Evidence of sensorineural hearing loss may indicate involvement of the labyrinth. Following surgery, 3% of operated ears have further impairment permanently due to the extent of the disease present or due to complications in the healing process. Patients should be counseled that there is a possibility of total loss of hearing in the operated ear. Also, with two-staged operations, the hearing will be worse after the first operation.
A labyrinthine fistula may occur in as many as 10% of patients with chronic ear infection due to cholesteatoma. A fistula should be suspected in a patient with longstanding disease with sensorineural hearing loss and/or vertigo induced by noise or pressure changes in the middle ear. Absence of a positive fistula test does not rule out this complication. Fine cut CT of the temporal bone should be obtained if this condition is suspected. The most common site of a labyrinthine fistula is the horizontal semicircular canal, although the basal turn of the cochlea is also at risk. The procedure of choice with this complication is a modified radical mastoidectomy, as discussed previously. Management of the matrix overlying the fistula depends on the infection status of the ear, the degree of hearing loss in the affected and nonaffected ear, the size and location of the fistula, and the surgeon’s experience. In an only hearing ear, matrix should be left intact over the fistula. Matrix should also be left over extensive fistulae of the vestibule or cochlea if hearing is normal. Matrix can be removed in a relatively dry, uninfected ear with a normal hearing opposite ear, and the fistula covered with bone pate or fascia.
Facial paralysis in patients with cholesteatoma requires immediate surgery. The paralysis may develop acutely secondary to infection or slowly from chronic expansion of the cholesteatoma. A temporal bone CT should be obtained to help localize the nerve involvement. The most common site of facial nerve involvement is at the geniculate ganglion due to disease in the anterior epitympanum. A simple mastoidectomy with a facial recess approach will expose the tympanic and mastoid portions of the facial nerve, while a middle fossa approach is required when there is petrous apex involvement. Removal of the cholesteatoma and infected material with decompression of the nerve usually results in the recovery of facial nerve function. Administration of intravenous antibiotics and high-dose steroids are also helpful. Iatrogenic injury to the nerve during surgery should be immediately repaired with decompression of the nerve proximal and distal to the site of injury.
Intracranial complications of cholesteatoma are potentially life-threatening. Infections such as a periosteal abscess, lateral sinus thrombosis and intracranial abscess occur in less than 1% of all cholesteatomas. Findings suggesting an impending intracranial complication include suppurative malodorous otorrhea, usually with chronic headache, pain and/or fever. The presence of mental status changes with nuchal rigidity or cranial neuropathies warrant neurosurgical consultation with urgent intervention. Epidural abscess, subdural empyema, meningitis and cerebral abscesses should be treated immediately prior to definitive otologic management of ear disease.
A brain hernia presents at a revision surgery as a meningoencephalocele or an encephalocele. It results from a defect in the tegmen tympani or tegmen mastoideum due to trauma from the drill at the time of the original surgery. This condition must be carefully inspected and repaired at the time of surgery.
Conclusion
The pathogenesis of cholesteatoma remains uncertain. The identification and behavior of the disease, however, is well described. For successful management of cholesteatomas, it is essential to possess a basic knowledge of the important anatomic and functional characteristics of the middle ear. Careful and thorough evaluations are the key to the early diagnosis and treatment of the disease. Early diagnosis and treatment can prevent complications and preserve hearing. Treatment of cholesteatoma is surgical with the primary goal to eradicate disease and provide a safe and dry ear. Surgical approaches must be customized to each patient depending on the extent of their disease. The surgeon must be aware of the serious and potentially life-threatening complications of cholesteatomas.
COCHLEAR IMPLANTS
History of Cochlear Implants
Volta, in the year 1790, became the first person to experience and publish the effects of electrical current on the auditory system. He inserted a metal rod in each ear and then subjected himself to approximately 50 volts of electricity. He reported that the sensation was that of receiving a blow to the head followed by the sound of thick soup boiling. Volta was followed by a string of scientists who continued to experiment with electricity and hearing over the next 167 years. It was Djourno and Eyries who reported the first stimulation of the acoustic nerve by direct application of an electrode in a deaf person (1957). The patient was undergoing an operation for cholesteatoma and the auditory nerve was exposed. An electrode was placed on the nerve and an induction coil and ground electrode were placed in the temporalis muscle. The coil could then be stimulated by currents produced by a second coil placed against the overlying skin. On subsequent experimentation with the patient reported hearing sounds like crickets or a roulette wheel when the second coil was applied. He was able to distinguish simple words and noted improvement of his speech reading ability. This initial implant was followed by a string of implantations performed by House, Doyle, Simmons, and others. Advances in microelectronics, biocompatible materials, and microscopic otologic surgery propelled House to produce the first single-channel implant in 1972 which stimulated the auditory nerve via the scala tympani. In 1984 the cochlear implant gained FDA approval for use in adults. This corresponded with the introduction of multichannel implants which significantly improved spectral perception and open-set speech understanding. The 1990’s saw significant improvements in speech processor designs. SPEAK and CIS speech processing strategies produced large improvements in recipient’s speech recognition. The late 1990’s and early 2000’s saw technologic improvements such as the peri-modiolar contour electrode, split electrodes, behind-the-ear processors, and implantation for children as young as 12 months.
Components of a Cochlear Implant
A cochlear implant consists of several components. All implants have microphones, external speech processors, signal-transfer hardware, transmitters, receivers, and electrodes. Each plays an important part in converting sound to an electrical stimulus. The microphone simply receives and transduces sound into an electrical representation. This is done in an analog (continuous) fashion.
The external speech processor and signal-transfer hardware shapes the electrical signal. This requires amplifying, compressing, filtering, and shaping. Amplification is necessary to increase some signal levels to the point that they can be used in the electrical circuits. Compression is a necessary second step of signal modulation. The normal human ear can hear gradations of sound intensity in a range of 120 dB. Persons with severe to profound hearing loss do not have this same range. In the high frequencies their dynamic range (the difference between their absolute threshold and painful sound) can be only 5 dB! The range in the lower frequencies is often 10-25dB. This means that significant compression of the sound energy must take place in order to render it useful. Thus, all cochlear implants employ gain control of one kind or another. These systems monitor the output voltage and adjust the ratio of compression to keep the output in a range where it provides useful, but not painful stimuli.
Filtering of the input signal is the next step. Frequencies between 100 Hz and 4000 Hz are generally those most important for understanding speech. Sound energy is analyzed using several different types of filters. This allows the unimportant frequencies to be removed and the frequencies of interest to be separately modified. Useful sound information is filtered into frequency bands. This information can then be analyzed for speech patterns and channeled to the appropriate portion of the electrode array.
The transmitter, or outer coil, is placed on the mastoid (usually held in place by magnets) and sends the processed signal to the receiver via radiofrequency. The receiver, surgically placed in a well over the mastoid, receives the signal and sends electrical energy to one or many electrodes in the array. The electrode array, which lies within the cochlea, delivers the electric signal to electrodes along its length. The electrical field generated at these locations serves to discharge the neural components of the auditory system. The eighth nerve then conveys this stimulus to the central nervous system which decodes and interprets the signal.
Just as important as any of the man-made components is the individual’s ability to adjust to, interpret and respond to the electrical stimulus. Length of time spent without sound stimulation of the auditory system, presence or absence of previous experience with sound, personal motivation, community or family support, and opportunities for rehabilitation have been shown to be important factors in achieving a good outcome. These factors likely are important in understanding significant differences in patient outcomes despite similar preoperative auditory deficits, surgical course, and cochlear implant hardware.
Types of Cochlear Implants
Cochlear implants differ in the way that they process sound and how they present electricity to the hearing nerve. Other than the speech processing strategies discussed below, there are two different ways of encoding sound information. The first form, analog coding, involves continuous coding of the sound signal with subsequent transfer to the receiver in multiple radio-frequency channels. Electrodes are continuously stimulated. The second form, digital coding, requires sampling of the sound waveform and assigning a number to these “bits” of information. These bits of information are then transferred to the receiver where they are decoded. Electrodes are stimulated in a pulse fashion. Interestingly, neither approach is 100% effective for all implant users. Recently, combining the two schemes has seen some success.
Cochlear implants can also be distinguished by their use of single vs. multiple channels, the number of electrodes, and their use of either monopolar or bipolar stimulation. The number of electrodes stimulated with different electrical stimuli determines the “channels” used. In other words, an implant may have multiple electrodes, but if the same information is presented to all the electrodes at one time they are essentially functioning as a single channel system. In contrast, multi-channel devices provide different information to several electrodes or groups of electrodes. Early implants had only one electrode (and one channel); recent advances have lead to the development of implants with multiple electrodes (22) and multiple channels (usually 4-8). Having more electrodes means that multiple channels can be localized to areas of the cochlea that are most responsive, and stray current that is stimulating adjacent structures (facial nerve, vestibular nerve) can be rerouted.
Cochlear implants can employ monopolar or bipolar stimulation. In a monopolar system there is only one ground electrode for all the others. The ground is usually located at or outside the round window. Thus an electrical field is created from the stimulated electrode to the ground. A bipolar arrangement is such that the ground for each electrode is much closer (adjacent to, or a few electrodes away). In the highly conductive environment of the inner ear, monopolar stimulation results in some limitations. As additional electrodes are stimulated with different streams (channels) of information the electrical fields created by stimulated electrodes may interfere with fields at other sites. This makes it difficult to stimulate more than one electrode at a time, or electrodes that are close together. The bipolar configuration was an attempt to limit this interaction by placing a ground near each electrode such that a smaller field would be created with less interference and more discrete stimulation. Once again, one approach does not achieve satisfaction with all patients. As a result, many implants offer both grounding methods.
Speech Processing Strategies
SPEAK:
CIS:
There are many different ways of processing the auditory signal for presentation at the level of the cochlear ganglia. The most commonly employed are the spectral peak (SPEAK), continuous interleaved sampling (CIS), and compressed analog stimulation. The SPEAK strategy is characterized by filtering sound into 20 different bands covering the range of 200 Hz to 10,000 Hz. Each filter corresponds to an electrode on the array. The outputs for each filter are analyzed and those channels of highest amplitude that contain speech frequencies are stimulated. The stimulus rate is equal to the period of the lowest frequency of speech (F0). The dominant speech frequency between 280 and 1000 Hz (F1) is then identified and the appropriate apical electrode is stimulated. The dominant speech frequency between 800 and 4000 Hz (F2) is then identified and the appropriate basal electrode is stimulated. Three additional high frequency filters measure input in the 2000-2800 Hz, 2800-4000 Hz, and >4000 Hz ranges. Stimulus is sent to apical electrodes (in order to take advantage of the greater incidence of ganglion cell survival at the apex of the cochlea). These channels provide additional cues for consonant perception and environmental sounds. Electrodes are stimulated sequentially, and at amplitudes specific for each frequency peak.
The continuous interleaved sampled (CIS) strategy is employed by the Clarion and MED-EL systems. This system works by filtering the speech into eight bands. The bands with the highest amplitude within the speech frequencies are subsequently compressed and their corresponding electrodes are stimulated. The CIS strategy uses high-rate pulsatile stimuli to capture the fine temporal details of speech.
The advanced combined encoder (ACE) strategy filters speech into a set number of channels and then selects the highest envelope signals for each cycle of stimulation. Stimulation is carried out in a very rapid fashion (much faster than the SPEAK strategy which stimulates at the rate of the lowest frequency of speech—180-300 cycles/second).
The simultaneous analog strategy (SAS) closely mimics the normal ear. All incoming sound is compressed and filtered into eight channels. These channels are then simultaneously and continuously presented to the appropriate tonotopic electrode. There is no effort to select for speech frequencies. Intensity is coded by either stimulus amplitude, rate or both.
The SAS strategy has met with limited success, whereas the SPEAK and CIS strategies have been relatively successful. It appears that no one system is effective for all recipients. For this reason, recent advances have made it possible for one cochlear implant to offer several speech processing strategies in the same implant. This allows the audiologist and patient to choose what strategy is best for that individual. Currently, the Nucleus systems are made to employ several processing strategies. These include spectral peak (SPEAK), advanced combined encoder (ACE), and continuous interleaved sampling (CIS). The Clarion systems use CIS to stimulate in a monopolar fashion as well as simultaneous analog stimulation (SAS). Medical Electronic (Med-El) produces a product (currently in USA clinical trials) with 12 electrode pairs suitable for deep insertion that relies on the CIS strategy with the most rapid stimulation rate of all implants. Recent advances in technologies have included the development of curved electrode arrays which are intended to more closely approximate the modiolas. Studies seem to indicate that electrodes closer to the basilar membrane need less current to stimulate the nerve and may improve spatial specificity of stimulation.
Indications for Cochlear Implantation
Cochlear implants are FDA approved for adults 18 years and older (no upper age limit) and children age 12 months to 17 years 11 months. Initially implants were only approved for adults who were postlingually deaf and had no improvement with high-powered hearing aids. This group of people has consistently been shown to be benefited by implantation. As more was learned about the benefits of cochlear implantation, the criteria were relaxed. Now, adult criteria include bilateral severe-to-profound sensorineural hearing loss with 70 dB pure tone average, little or no benefit from hearing aids (must attempt binaural high-powered hearing aids for at least 6 months), and psychological suitability. Audiologic examination should show word discrimination scores less than 40% in the best aided condition. The patient should have no anatomical deformity that would preclude implantation success. Finally, the patient should have no physical condition that would preclude a general anesthetic.
Pediatric implantation is indicated in children 12 months or older with bilateral severe-to-profound sensorineural hearing loss with pure tone averages of 90 dB or greater in the better ear. The child must have had no appreciable benefit with hearing aids (evaluated with parental survey when younger than 5 and 30% or less on sentence recognition tests under best-aided conditions when 5 years old or older). Children must tolerate wearing hearing aids for a period (as all cochlear implants have external components), and show some aided communication ability. Children must be enrolled in educational programs that support aural/oral learning and have no medical contraindications. Parents must be highly motivated and have reasonable expectations.
Contraindications for Cochlear Implantation
Not all patients with sensorineural hearing loss are good candidates for cochlear implantation. For example, patients with pure tone thresholds greater than 90 dB with residual hearing through 2000 Hz often do better with hearing aids than with implantation. Computed tomography findings may also preclude implantation. The absence of the cochlea (Michel deformity), and a small internal auditory canal (associated with cochlear nerve atresia) are contraindications to implantation on that side. Other forms of dysplasia are not necessarily contraindications. However, when implantation of a dysplastic cochlea is to be undertaken informed consent is especially important. Cochlear implants in these patients are associated with increased risk of poor result, CSF leak, and meningitis.
The presence of active middle ear disease is a contraindication to surgery. This process should be treated and resolved before implantation. In a study by Luntz otitis-prone patients were treated by protocol (antibiotics, PETs, etc.) before surgery and then implanted (often with PETs in place). No delay was necessary when compared with patients who were not otitis-prone. Several were noted to have inflamed middle ear mucosa on implantation which required removal in order to identify the round window, but did very well with few postoperative episodes of otitis. Children with a history of chronic suppurative otitis media were implanted in a study by El-Kashlan without demonstrable early or late complications. Patients with a history of canal wall down mastoidectomy may need surgery to reconstruct the posterior canal wall or close off the canal before implantation.
Meningitis may lead to hearing loss and ossification of the cochlea. Labyrinthitis ossificans is usually identifiable on CT scan (brightly lit cochlea with obliteration of the basal cochlear duct) and is a relative contraindication when there is a patent contralateral basal turn. MRI is often better at delineating patency of the cochlea and should be pursued if there is any question. Very young children with hearing loss after meningitis should be followed with CT/MRI until they reach implantable age. Early implantation may be indicated if evidence of ossification is noted. Adults and children with acute meningitis should be treated with steroids to avoid hearing loss. Those that do sustain hearing loss secondary to meningitis should be observed for 6 months before implantation due to the substantial number of patients that will regain their hearing in at least one ear. Advanced otosclerosis can also cause ossification of the basal turn of the cochlea. This finding is most often noted on CT scan. This is not a contraindication as long as the surgeon is prepared to perform a drill out or pursue implantation into the scala vestibuli. Patients with otosclerosis can achieve excellent results from implantation.
A diagnosis of neurofibromatosis II (history of progressive hearing loss and suggestive MRI findings), mental retardation, psychosis, organic brain dysfunction, and unrealistic expectations may also be contraindications.
Work-up for a Patient Seeking Cochlear Implantation
• Audiologic examination with binaural amplification
• CT scan/MRI of temporal bones
• Trial of high-powered hearing aids
• Psychological evaluation
• Medical evaluation
• Any workup necessary to discover etiology of hearing loss
Surgical Procedure
The surgical procedure to implant the receiver and electrode array is most often a day-surgery with the patient being discharged shortly after completion of the implantation. Implanting the better or worse-hearing ear is a decision reached by the physician and the patient. The patient should understand the risk of losing all residual hearing in the implanted ear. One recent study by Chen showed no long-term advantage to implanting the patient’s better ear. Thus, many surgeons opt to implant the worse ear and have the patient continue to wear an aid in the best hearing ear.
The surgical procedure begins after the patient receives a general anesthesia. The patient’s head is shaved over the post-auricular area. The extent of hair removal depends on the incision to be used–generally four fingerbreadths above and behind the ear is sufficient. The patient is then prepped and draped in a fashion similar to other otologic procedures. A dummy receiver is placed over the skin and positioned approximately 1 cm posterior to the auricle. A postauricular incision is made 1-2 cm posterior to the implant. Several incisions have been proposed and include a large C-shaped incision, a 4-5 cm superior elliptical extension of the routine postauricular incision, a small 4-5 cm straight incision posterior and an incision posterior-superior to the auricle (minimally invasive procedure introduced by the Cochlear Company). The skin flap is elevated followed by the creation of an anteriorly-based temporoparietal fascia flap. The temporalis musculature and overlying fascia are left intact. A subperiosteal pocket is created medial to the temporalis muscle for placement of the ground electrode. A circular depression is then drilled in the temporal bone cortex superior and posterior to the area to be drilled for access to the round window. Tunnels are often drilled into the surrounding bone in order to place anchor sutures over the receiver.
A complete mastoidectomy is performed with minimal saucerization. A shelf of mastoid cortex can be helpful when securing the array and tucking the excess grounding wire. The facial recess is opened, taking care to avoid injury to the chorda tympani and facial nerves. The round window niche is inspected. A cochleostomy is then drilled over the basal turn of the cochlea just anterior/inferior to the round window. This is carried down to endosteum of the cochlea. The endosteum is then opened using a straight pick. The electrode array is then carefully inserted through the fenestra into the scala tympani. An inserting claw or jeweler’s forceps may be used to advance the electrode array. Excess force should not be used, as the array can easily buckle and cause damage to the internal components. A deep insertion is desired in order to place the electrodes closer to the apex where the highest concentrations of surviving ganglion often are found. A small amount of connective tissue is then packed around the electrode array at the cochleostomy site in order to seal the opening. Care is taken to avoid accidental removal of the array once placed. The ground electrode is tucked into the sub-periosteal pocket and the wound is closed in several layers. No drains are placed. A bulky mastoid dressing is applied. The wound is given several weeks to heal before use of the external processor is attempted. The external processor is held in place by magnetic attraction to the magnet in the implanted receiver.
In patients who have a history of meningitis leading to hearing loss, labyrinthitis ossificans may have caused obliteration of the scala tympani. In this case, the array can be placed into the scala vestibuli. Often the ossification is incomplete and if the surgeon drills forward along the basal coil for 4-5mm the scala tympani will be identified. Care must be taken to avoid injury to the carotid artery which lies just anterior to the cochlea. In some cases of cochlear dysplasia CSF gushers have been encountered. This is managed by allowing the pressured fluid to drain off, and then proceeding with insertion as per routine.
Postoperative Management
The surgical complication rate after cochlear implantation is estimated to be only 5%. The most common problems are wound infection and wound breakdown. Rarely, extrusion of the device, facial nerve injury, bleeding, CSF leaks and meningitis can occur. Device-related complications include intracochlear damage, slippage of the array, breakage of the implant, and improper or inadequate insertion. Postoperative infection of the surgical site was treated by prolonged courses of postoperative antibiotics by Yu, et al with excellent results. They suggest that a long course of antibiotics and limited I&D will treat the vast majority of wound infections without the need to remove the implant. Those patients that did not respond to this treatment protocol were often found to be immunocompromised. Steenerson reported a 75% incidence of postoperative vertigo, but indicated that these patients did well after undergoing vestibular therapy. Other series do not show as high an incidence, nor the need for vestibular rehabilitation postoperatively. Stimulation of the facial or vestibular nerve by stray electrical current from electrodes outside or near the round window has also been reported. This is usually addressed by “turning off” the responsible electrodes and moving the electrical stimulation to electrodes located within the cochlea.
Recent reports of increased incidence of meningitis in cochlear implant recipients have prompted the CDC to recommend vaccination of implanted or soon to be implanted patients. Children less than 2 years old who have implants should receive pneumococcal conjugate vaccine (Prevnar). Children with implants 2 years and older who have completed the conjugate series should receive one dose of the pneumococcal polysaccharide vaccine (Pneumovax 23 or Pnu-Imune 23). Children with implants between 24 and 59 months who have never received vaccination should receive two doses of pneumococcal conjugate vaccine two months apart and then one dose of pneumococcal polysaccharide vaccine at least two months later. Finally, persons age 5 years and older with cochlear implants should receive one dose of pneumococcal polysaccharide vaccine.
Although the incidence of device failure is very low, occasionally removal of the implant and reimplantation is necessary. These patients do surprisingly well. Alexiades, et al. showed that patients did as well or better after reimplantation (in the same ear) as with their first implant. Thus a history of implantation is not a contraindication to another cochlear implant. Long-term electrical stimulation from a cochlear implant has raised concern for damage to the auditory nerve. However, cochlear implants typically discharge less than one microcoulombs per cm2 of electrode surface and long-term studies have shown no detrimental effects. In fact, studies following patients for up to 13 years show no decline in function. This finding is still true when the study population includes those that have been implanted multiple times.
Research looking at cochlear implants under many environmental strains has shown them to be reliable and safe. Backous, et al showed them to be stable when exposed to extreme barometric pressure changes (as experienced when scuba diving). MRI exposure should be avoided generally, but may be pursued when necessary.
Postoperative Rehabilitation
Unless intensive postoperative rehabilitation is undertaken, cochlear implantation is likely to provide little benefit. Each patient’s need for rehabilitation is different based on pre-operative auditory experience. For the prelingually deaf patient, auditory and speech training are imperative if they are to improve their communication abilities. Postlingually deaf patients often need training in more complex listening skills. Cochlear implants in children are successful when the implantation is followed by a intensive treatment by a multidiscipline rehabilitation team. The goal of a pediatric rehabilitation team is to enable the hearing-impaired child to be able to learn passively from his environment. The rehabilitation must address both receptive language skills as well as expressive language abilities. A structured program with dedicated team members is integral to a successful cochlear implant program.
Results of Cochlear Implantation
Cochlear implantation is really the only effective way of treating patients with profound sensorineural hearing loss who do not benefit from hearing aids. Although the perception of a successful implantation might vary from patient to patient, the primary goal of implantation has always been improved speech perception. Since implantation began, physicians have noted a wide range of outcomes. Some patients find little benefit after implantation and may even find the stimulation annoying. Others are able to function normally even without visual cues. Still others are able to listen to and enjoy music. Years of research has given us a better understanding of what variables might influence the results of implantation.
The age of onset of deafness, as well as the length of time since the onset of hearing loss has both been shown to influence outcomes. Several studies have shown that patients who were prelingually deafened show the poorest outcome. Prelingually deaf children implanted before age 6 appear to be able to “catch up” to implanted postlingually deaf children within 2-5 years. These children, like their postlingual counterparts, are able to achieve open-set speech discrimination. Several studies have shown that implantation at an earlier age results in earlier achievement of open-set speech discrimination. Govaerts, et al. showed that 90% of those implanted before age 2 were integrated into mainstream education whereas only 20-30% of those implanted after age 4 were ever integrated. These results are seen in children who are enrolled in aural/oral educational programs and who use oral language as their primary communication modality. The performance of implanted children is far better than those with equal hearing deficits who rely on vibrotactile devices or hearing aids. Generally, implantation of prelingually deaf adolescents and adults is significantly less successful, though results vary widely.
Most authors now believe that the shorter the period of auditory deprivation, the faster and more complete will be the achievement of open-set speech discrimination. This has been shown to be true in the adult population, as well with children. Those patients who are implanted within a short time seem to retain the plasticity of the auditory system better than those who have been deaf for a period of years. Sharma, et al. compared children implanted after different periods of deafness. He showed that children with the shortest amount of time spent without auditory stimuli regained normal cortical responses more rapidly than all others. Specifically, those with 3.5 years of deafness or less showed age-appropriate P1 latencies (a marker of plasticity) after only 6 months of stimulation with a cochlear implant. The length of time required to reach age-appropriate latencies increased with increasing length of auditory deprivation. After age 7 plasticity was greatly reduced.
Waltzman, et al. studied the long-term effects of cochlear implants in children. They followed the children after implantation for five to fifteen years and documented speech perception scores, device extrusion rates, and implant viability. He showed that implantation resulted in significant improvement of patient’s speech perception and that this benefit remained stable (often improving) over the long-term. For the vast majority of his study group this resulted in assimilation into mainstream education. There was no significant incidence of device extrusion or migration and even when device failure necessitated reimplantation, long-term performance was not decreased.
Recent studies looking at the economics of cochlear implants show cochlear implantation improves patient’s quality of life and is cost-effective even in elderly patients (>50 years old). Implantation results in significant benefit to the society as a whole, and to the individual. Unfortunately, cochlear implantation is more often a money-losing effort for everyone involved with implantation. Hospitals and physicians, as well as the other members of the rehabilitation team often find themselves without funding and support.
Future Directions
Partial insertion cochlear implantation has been proposed as treatment for those patients who have residual low-frequency hearing with high-frequency sensorineural hearing loss. The speech processor is coupled with a hearing aid and thus provides maximal aided hearing. The risk of such surgery is loss of the remaining hearing in that ear. Other implant strategies include brainstem implantation for those without an intact cochlear nerve. Good results have been reported.
Nucleus products now come equipped for intraoperative testing. This allows the audiologist to map the patient’s electrode array while the patient is asleep. This is especially useful for infants and small children who are often not cooperative with conventional mapping techniques. Intraoperative repositioning is also a possibility if the mapping shows poor responses.
Bilateral implantation with the possibility of binaural hearing is currently being studied. Gantz showed that most patients with bilateral implants perform better on sound localization, but only some do better with auditory performance in noise (at one year). Despite this, most studies report increased satisfaction with two implants. At least one company is proposing a system with only one processor and receiver but implanted electrodes in both ears. Despite these results, the cost of a second implant is prohibitive. Summerfield argued that the quality of life likely to be gained (across society as a whole) by unilateral implantation is higher, per unit of expenditure, than with bilateral implants.
Implantation for patients with asymmetric sensorineural hearing loss may soon be approved. Cochlear implants are expected to help these patients with sound localization and speech comprehension in noise.
The Cochlear Company is currently testing a new “Softip” electrode array which is advanced off a stylet after traversing the straight section of the basal turn. The array then curls around the modiolus until fully inserted. The technique has been shown to cause less trauma to the basilar membrane and intracochlear structures in preliminary studies. The Cochlear Co. is also marketing its new “minimally invasive” approach which allows for implantation through a small (4-5cm) incision over the post auricular area which does not require shaving of the hair in that area. Warm response is reported by those performing this approach.
Conclusion
Cochlear implantation is no longer experimental. It is the treatment of choice for children and adults with severe-to-profound hearing loss. Significant gains in open-set speech recognition have been demonstrated by most of those who undergo implantation. Early implantation, whether in pre or postlingual patients, has shown to be effective at moving an otherwise marginalized segment of society into the mainstream. Implantation is cost-effective and results in high patient satisfaction. Although cochlear implants are still a rough and awkward imitation of our natural sense, they offer hope to thousands who must otherwise live in a silent world.
OTOACOUSTIC EMISSION
The primary purpose of otoacoustic emission (OAE) tests is to determine cochlear status, specifically hair cell function. This information can be used to (1) screen hearing (particularly in neonates, infants, or individuals with developmental disabilities), (2) partially estimate hearing sensitivity within a limited range, (3) differentiate between the sensory and neural components of sensorineural hearing loss, and (4) test for functional (feigned) hearing loss. The information can be obtained from patients who are sleeping or even comatose because no behavioral response is required.
The normal cochlea does not just receive sound; it also produces low-intensity sounds called OAEs. These sounds are produced specifically by the cochlea and, most probably, by the cochlear outer hair cells as they expand and contract. The presence of cochlear emissions was hypothesized in the 1940s on the basis of mathematical models of cochlear nonlinearity. However, OAEs could not be measured until the late 1970s, when technology created the extremely sensitive low-noise microphones needed to record these responses.
The 4 types of otoacoustic emissions are as follows:
- Spontaneous otoacoustic emissions (SOAEs) – Sounds emitted without an acoustic stimulus (ie, spontaneously)
- Transient otoacoustic emissions (TOAEs) or transient evoked otoacoustic emissions (TEOAEs) – Sounds emitted in response to an acoustic stimuli of very short duration; usually clicks but can be tone-bursts
- Distortion product otoacoustic emissions (DPOAEs) – Sounds emitted in response to 2 simultaneous tones of different frequencies
- Sustained-frequency otoacoustic emissions (SFOAEs) – Sounds emitted in response to a continuous tone
Pure-tone (PT) audiometry measures throughout the outer ear, middle ear, cochlea, cranial nerve (CN) VIII, and central auditory system. However, OAEs measure only the peripheral auditory system, which includes the outer ear, middle ear, and cochlea. The response only emanates from the cochlea, but the outer and middle ear must be able to transmit the emitted sound back to the recording microphone. OAE testing often is used as a screening tool to determine the presence or absence of cochlear function, although analysis can be performed for individual cochlear frequency regions. OAEs cannot be used to fully describe an individual’s auditory thresholds, but they can help question or validate other threshold measures (eg, in suspected functional [feigned] hearing loss), or they can provide information about the site of the lesion.
Using current technology, most researchers and clinicians find a correlation between frequency-specific analysis of TOAEs/DPOAEs and cochlear hearing loss. However, at this juncture, the correlation cannot fully describe auditory threshold. Naturally, a correlation would not be expected for noncochlear hearing loss.
Recording
Insert a probe with a soft flexible tip in the ear canal to obtain a seal. Use different probes for neonates and adults; the probes are calibrated differently because of the significant difference in ear canal volume. The smaller ear canal results in a higher effective sound pressure level (SPL), thus a different probe is used to correct for the difference.
Multiple responses are averaged. All OAEs are analyzed relative to the noise floor; therefore, reduction of physiologic and acoustic ambient noise is critical for good recordings. Because no behavioral response is required, OAEs can be obtained even from patients who are comatose. For a quiet and cooperative patient, recordings usually require less a few minutes per ear. For an uncooperative or noisy patient, recordings may take significantly longer or may be impossible to obtain on a given visit.
Recording Parameters
For all OAEs, an optimal probe fit is critical. Complete information on recording and interpreting OAEs is beyond the scope of this article; for discussions that are more comprehensive, please see the bibliography.
Spontaneous otoacoustic emissions
This nonevoked response usually is measured in narrow bands ( <30 Hz bandwidth) of frequencies recorded in the external ear canal. No stimulus is required. Obtain multiple recordings to ensure replicability and to distinguish the response from the noise floor. SOAE recordings usually span the 500- to 7000-Hz frequency range.
Transient otoacoustic emissions
Clicks are the most commonly used stimuli, although tone-burst stimuli may be used. Most commonly, 80- to 85-dB SPL stimuli are used clinically. The stimulation rate is less than 60 stimuli per second. TOAEs are generally recorded in the time domain over approximately 20 milliseconds. Alternating responses are stored in alternating computer memory banks, A and B. Data that correlate between the 2 memory banks are considered a response. Data that do not correlate are considered noise. When present, TOAEs generally occur at frequencies of 500-4000 Hz. Data in the time domain then are converted to the frequency domain, usually in octave band analysis.
Distortion product otoacoustic emissions
Stimuli consist of 2 pure tones at 2 frequencies (ie, f1, f2 [f2>f1]) and 2 intensity levels (ie, L1, L2). The relationship between L1-L2 and f1-f2 dictates the frequency response. An f1/f2 ratio yields the greatest DPOAEs at 1.2 for low and high frequencies and at 1.3 for medium frequencies. To yield an optimal response, set intensities so that L1 equals or exceeds L2. Lowering the absolute intensity of the stimulus renders the DPOAEs more sensitive to abnormality. A setting of 65/55 dB SPL L1/L2 is frequently used. Responses are usually most robust and recorded at the emitted frequency of 2 f1–f2; however, they generally are charted according to f2 because that region approximates the cochlear frequency region generating the response.
Prerequisites for obtaining otoacoustic emissions
- Unobstructed outer ear canal
- Seal of the ear canal with the probe
- Optimal positioning of the probe
- Absence of middle ear pathology: Pressure equalization (PE) tubes alone probably will not interfere with results. However, if emissions are absent, results should be interpreted with caution.
- Functioning cochlear outer hair cells
- A quiescent patient: Excessive movement or vocalization may preclude recording.
- Relatively quiet recording environment: A sound booth is not required, but a noisy environment may preclude accurate recording.
INTERPRETATION
Spontaneous otoacoustic emissions
In general, SOAEs occur in only 40-50% of individuals who have normal hearing. For these adults, the range is about 30-60%; in neonates with normal hearing, the range is approximately 25-80%. SOAEs generally are not found in individuals with hearing thresholds worse than 30 dB HL. Therefore, the presence of SOAEs usually is considered a sign of cochlear health, but the absence of SOAEs is not necessarily a sign of abnormality.
When present in humans, SOAEs usually occur in the 1000- to 2000-Hz region; amplitudes are between -5 and 15 dB SPL. Some individuals have multifrequency SOAEs over a broader frequency range.
SOAEs typically are bilateral rather than unilateral. If unilateral, they are more likely to be present in the right rather than in the left ear. SOAEs occur more often in females than in males (across all ages).
Usually, SOAEs are not associated with tinnitus. Because tinnitus often occurs in conjunction with cochlear abnormality, SOAEs usually are absent. SOAEs are seldom used clinically to screen hearing. The absence of SOAEs does not imply abnormal auditory function, as indicated above.
High-level SOAEs may occur. These emissions can be heard by others. Objective tinnitus is usually a misnomer because the patient often cannot hear these noises. Such emissions are very uncommon but may coexist with sensory hearing loss. High-level SOAEs are more common in children than in adults.
Transient otoacoustic emissions
In the clinic, TOAEs commonly are used to screen infant hearing, to validate behavioral or electrophysiologic auditory thresholds, and to assess cochlear function relative to the site of the lesion. By definition, TOAEs are recorded only in response to very short or transient stimuli. Therefore, the stimulus has limited frequency specificity, and the TOAE emanates from a relatively broad cochlear region. However, current analysis techniques allow the response to be separated into various frequency bands for analysis.
In general, the presence of a TOAE in a particular frequency band suggests that cochlear sensitivity in that region is approximately 20-40 dB HL or better, depending on the study cited. Most clinicians use the presence of a TOAE in a particular octave band to suggest that hearing sensitivity should be 30 dB HL or better, unless a functional or neural component is present.
Distortion product otoacoustic emissions
The relative merits of TOAEs and DPOAEs are widely discussed. Essentially, DPOAEs allow greater frequency specificity and can be used to record at higher frequencies than TOAEs. Therefore, DPOAEs may be as particularly useful for early detection of cochlear damage as they are for ototoxicity and noise-induced damage. However, large-scale comparative studies of TOAEs and DPOAEs in these groups of patients currently are lacking. Reliability of DPOAEs is greatest above 1000 Hz.
For infant hearing screening, both DPOAEs and TOAEs are used. TOAEs have been used clinically for a longer period and are more established regarding association with behavioral audiometric thresholds.
Depending on the methodology employed, DPOAEs often can be recorded in individuals with mild-to-moderate hearing losses for whom TOAEs are absent; however, the accuracy of DPOAEs in estimating actual hearing sensitivity is not fully resolved (research continues in this area). DPOAEs frequently correspond to the audiometric configuration of a cochlear hearing loss, which is helpful in some patients.
Sustained-frequency otoacoustic emissions
SFOAEs are responses recorded to a continuous tone. Because the stimulus and the emission overlap in the ear canal, the recording microphone detects both. Therefore, interpretation depends on reading a complicated series of ripples in the recording. At present, SFOAEs are not used clinically.
Factors that can Affect Otoacoustic Emissions
Nonpathologic problems that can cause absence of OAEs
- Poor probe tip placement or poor seal: Most current equipment alerts clinicians to these problems.
- Standing waves: Most current equipment alerts clinicians to standing waves.
- Cerumen occluding the canal or blocking a probe port
- Debris and foreign objects in the outer ear canal
- Vernix caseosa in neonates: This is common immediately after birth.
- Uncooperative patient: Usually, recordings simply are not obtained.
Pathologic problems that can cause absence of OAEs
Outer ear
- Stenosis
- External otitis
- Cyst
- Abnormal middle ear pressure
Tympanic membrane – Perforation of the eardrum (PE tubes do not necessarily prevent good recordings.)
Middle ear
- Otosclerosis
- Middle ear disarticulation
- Cholesteatoma
- Cyst
- Bilateral otitis media: To record OAEs, the cochlear response must be able to travel efficiently through the middle ear and tympanic membrane to the recording microphone in the ear canal. Even in the presence of normal cochlear function, OAEs generally are absent in the presence of otitis media. OAE testing is best conducted after the otitis media has cleared. If the patient cannot be tested later, when the otitis has cleared, no harm exists in attempting to record OAEs. If OAEs are present (as in a very small percentage of patients with otitis media), that information could be useful. If they are absent (as in most patients with otitis media), no conclusions about cochlear function can be drawn.
Cochlea
- Exposure to ototoxic medication or noise exposure (including music): OAE changes may precede threshold changes in the conventional frequency range.
- Any other cochlear pathology
Conditions that do not affect OAEs
- CN VIII pathology: If CN VIII pathology also affects the cochlea (eg, vestibular schwannoma that decreases cochlear vascular supply), OAEs are affected.
- Central auditory disorder
Conditions that elicit abnormal OAEs and normal behavioral thresholds
- Tinnitus: OAEs may be abnormal in the frequency region of the tinnitus.
- Excessive noise exposure
- Ototoxicity
- Vestibular pathology
Conditions that elicit normal OAEs and abnormal behavioral thresholds
- Functional hearing loss
- Attention deficits
- Autism
- Possibly, inner hair cell damage but normal outer hair cells (reported for animals but no human reports yet)
- Auditory neuropathy: This includes central auditory nervous system dysfunction and CN VIII auditory dysfunction.
AUDITORY NEUROPATHY
The advent of OAE recordings opened a new area of auditory investigation in auditory neuropathy. Although auditory neuropathy is not a new disorder, OAEs have triggered numerous new studies. Therefore, a more complete listing is provided for this disorder.
Classic auditory neuropathy is characterized by the presence of OAEs, abnormal ABR findings, and, often, absent or abnormal behavioral responses to sound. (OAEs may be absent and an auditory neuropathy still may exist if concomitant cochlear disorder is present.) ABR abnormalities consistent with auditory neuropathy include absence of all ABR waveforms or prolonged interpeak latencies. A large cochlear microphonic sometimes is observed on the ABR recordings for these patients. The patient with auditory neuropathy may have any type of audiometric configuration, but rising or flat configurations are most common. Often, the patient’s word recognition is disproportionately poor relative to PT thresholds. Listening in noise usually is very difficult. Hearing may fluctuate. Over time, it may stabilize, improve, or progress to profound hearing loss. If the etiology is known, a more accurate prognosis may frequently be given; however, the disorder can be idiopathic.
The cause of auditory neuropathy sometimes is unknown; however, the following conditions may be associated with pediatric auditory neuropathy:
- Hyperbilirubinemia
- Neurodegenerative diseases
- Neurometabolic diseases
- Demyelinating diseases
- Hereditary motor sensory neuropathologies (eg, Charcot-Marie-Tooth diseases with deafness)
- Inflammatory neuropathy
- Hydrocephalus
- Severe and/or pervasive developmental delay
- Ischemic-hypoxic neuropathy
- Encephalopathy
- Meningitis
- Cerebral palsy
ANATOMY AND PHYSIOLOGY UNDERLYING OTOACOUSTIC EMISSIONS
Because OAEs may be new to some clinicians, a brief review of the relevant anatomy and physiology is provided.
When sound is used to elicit an emission, it is transmitted through the outer ear, where the auditory stimulus is converted from an acoustic signal to a mechanical signal at the tympanic membrane and is transmitted through the middle ear ossicles; the stapes footplate moves at the oval window, causing a traveling wave in the fluid-filled cochlea. The cochlear fluid’s traveling wave moves the basilar membrane; each portion of the basilar membrane is maximally sensitive to only a limited frequency range. The arrangement is a tonotopic gradient. Regions closest to the oval window are more sensitive to high-frequency stimuli. Regions further away are most sensitive to lower-frequency stimuli. Therefore, for OAEs, the first responses returned and recorded by the probe microphone emanate from the highest-frequency cochlear regions because the travel distance is shorter. Responses from the lower-frequency regions, closer to the cochlear apex, arrive later.
When the basilar membrane moves, the hair cells are set into motion and an electromechanical response is elicited, while an afferent signal is transmitted and an efferent signal is emitted. The efferent signal is transmitted back through the auditory pathway, and the signal is measured in the outer ear canal. As described above, the responses from the high-frequency region arrive first, progressively followed by responses from lower-frequency regions.
Outer hair cells are located in the organ of Corti on the basilar membrane. These hair cells are motile; an electrochemical response elicits a motoric response. The 3 rows of outer hair cells have stereocilia arranged in a W formation. The stereocilia are linked to each other and, therefore, move as a unit. These are the outer hair cells believed to underlie OAE generation.
Complications of Stapes Surgery
Introduction
Otosclerosis is a bone disease only seen in the otic capsule. It causes hearing loss which may be conductive, mixed, or sensorineural hearing loss. Toynbee first described the condition in 1860 as causing a hearing loss by fixation of the stapes. In 1894, Politzer referred to the fixation of the stapes as otosclerosis. Siebenmann revealed on microscopic examination that the lesion seemed to begin as spongification of the bone and termed the process otospongiosis.
Otosclerosis clinically presents with progressive hearing loss. If the otospongiotic change primarily involves the stapes, then the hearing loss is conductive. The fissula ante fenestram is the most common area for stapedial fixation. The process may progress to involve the entire footplate or may continue anteriorly toward the cochlea, causing a sensorineural hearing loss.
Otosclerosis is an autosomal-dominant hereditary disease. It has varialble penetrance and expression. Women are effected by otosclerosis 2:1. Hearing loss usually begins in the late teens or early twenties, but may occur later. The prevalence of otosclerosis varies with race and its expression. The disease is found between 7-10% of cadevaric temporal bones in Caucasians. Clinical otosclerosis is rare in blacks, Asians, and Native Americans.
Histopathology
Early lesions tend to begin near the fissula ante fenestram. They begin as connective tissue replacing bone, which in turn is remodeled by osteocytes resulting in disorganized bone with enlarged marrow spaces. When this process involves the mobility of the stapes, a conductive hearing loss results. Occasionally, the lesion can involve only the cochlea, causing an isolated sensorineural hearing loss.
Evaluation
History
Patient history is one of the most important aspects of the evaluation of the otosclerosis patient. Hearing loss usually gradual in onset and slowly progressive over several years.
Approximately 70% of otosclerosis cases are bilateral. Typically, hearing loss becomes apparent in the late teens or twenties, but may not become apparent to the patient until age 30 or 40 years. As is typical with conductive hearing loss, patients will report difficulty hearing conversation while chewing and may hear better in noisy rooms because people speak louder in noisy surroundings. Unilateral hearing loss is less noticeable to the patient and results in difficulty with direction of sound and in noisy rooms. Family history is usually positive for hearing loss often with surgical correction.
Physical Examination
Physical examination includes the following:
1. Otoscopy (often with the operating microscope)- look for Schwartze sign which is a red blush over the promontory or the area anterior to the oval window
2. Pneumo-otoscopy- evaluates for middle ear effusion or small perforation
3. Tuning fork exam- may confirm or dispute the finding of a conductive hearing loss on audiometry
Audiometry
The standard audiometric evaluation includes air conduction, bone conduction, and speech audiometry. Additionally, the immittance audiometry battery consists of tympanometry, static compliance, and acoustic reflex testing. The middle ear pressure is not affected by otosclerosis, and, therefore, the tympanogram is normal with a distinct peak that occurs with the normal range. The peak may be lower (As) than normal in the presence of a healthy appearing tympanic membrane, thus alerting the examiner to the possible diagnosis. Acoustic reflexes are a sensitive measure of the movement of the stapes, which in the presence of advanced otosclerosis, the reflex will be absent.
Surgery
As with all surgery, surgical options for treatment of otosclerosis involves risk. Total sensorineural hearing loss occurs in about 0.2% of cases, but the patient is told that there is a less than 2% chance of further hearing loss and a less than 1% chance of losing all hearing in the operated ear. Dizziness may occur postoperatively also. Dizziness is usually transient and brief. However, it may persist for a short period of time and rarely could be permanent. The possibility of facial palsy should be mentioned as well.
Surgical techniques include stapedotomy or stapedectomy performed with either a laser or microdrill. Please refer to otology texts for further description of surgical techniques.
Multiple problems can be encountered during stapes procedures. These are listed below:
– Exposed, Overhanging Facial Nerve
An exposed facial nerve occurs in approximately 9% of stapes procedures. It may block access to the footplate, making the completion of the procedure impossible. Gentle retraction of the nerve superiorly with a small suction while the drill or laser is used to create the fenestra is possible. If the prosthesis is touching the facial nerve it generally does not create a problem for postoperative hearing or facial function.
-Fixed Malleus
A fixed malleus is a rare problem, but it should be checked for in every procedure. When this occurs, the sound conduction can be reestablished with an incus replacement prosthesis or a total ossicular replacement prosthesis and tragal cartilage.
-Solid or Obliterated Footplate
A solid or obliterated footplate can be managed with a microdrill to create a fenestra. It was a greater problem in the past when a total stapedectomy was performed.
-Floating Footplate
A floating footplate rarely occurs when using the laser or microdrill, especially when the crura are left in place until after the prosthesis is placed. Management includes carefully drilling a small hole in the promontory at the inferior edge of the footplate followed by using a small hook to gently elevate the footplate out of the oval window.
Perilymph Gusher
A perilymph “gusher” is the profuse flow of cerebrospinal fluid (CSF) immediately on opening the vestibule. It is rare with a reported incidence of 0.03% and is most often associated with congenital footplate fixation in the pediatric population. The etiology of this CSF leak is thought to be due to either a widened cochlear aqueduct or a defect in the fundus of the internal auditory canal. Management involves placement of a tissue graft over the oval window and completion of the procedure, if possible, rather than packing the ear and terminating the surgery. Placement of a lumbar drain can also be used to reduce CSF pressure.
Tympanic Membrane Perforation
A tympanic membrane perforation may occur during elevation of the tympanomeatal flap. Perforation does not preclude completion of the operation. Repair involves myringoplasty with either synthetic material or autologous tissue.
Chorda Tympani Nerve Damage
Damage to the chorda tympani nerve may occur in up to 30% of cases secondary to stretching and mobilization of the nerve during removal of the posterosuperior bony canal wall. Sequelae include complaints of temporary dry mouth, tongue soreness, or a metallic taste that
usually subsides in 3 to 4 months. Symptoms are less severe with complete sectioning of the nerve rather than stretching or partial tearing.
Intraoperative Vertigo
When the prosthesis is too long, vertigo may result. Vertigo also may be induced when checking the mobility of the prosthesis. In this situation, a shorter prosthesis generally corrects this problem. If the vertigo does not resolve, the prosthesis can be removed and replaced with a 0.25-mm shorter piston.
Postoperative Complications
Sensorineural Hearing Loss
The most devastating complication of stapes surgery is sensorineural hearing loss which occurs in less than 1% of cases. Sensorineural hearing loss may be mild or isolated to high frequencies. When sensorineural hearing loss is suspected, prednisone is started immediately and tapered.
Serous labyrinthitis is common after stapedectomy due to inner ear inflammation. Patients may exhibit mild unsteadiness, positional vertigo, and/or a slight decrease in high-frequency hearing. The above symptoms typically resolve within several days to weeks, correlating with the resolution of the serous labyrinthitis.
Postoperative reparative granuloma is rare and has previously been recognized as a cause of sensorineural hearing loss after stapedectomy. Patients present with initial improvement in hearing followed by a gradual or sudden deterioration 1 to 6 weeks postoperatively. Vertigo can also occur and clinical examination often reveals a reddish discoloration in the posterosuperior quadrant of the tympanic membrane. Treatment consists of prompt recognition and removal of the granuloma from around the oval window.
Vertigo
Mild vertigo or dizziness is fairly common and occurs in about 5% of cases. It is rarely prolonged or severe and usually lasts for a few hours, subsiding rapidly. Management is usually not necessary or may be supportive only.
Facial Paralysis
Rarely, delayed onset of facial palsy occurs postoperatively usually occurring in the 5 day post-operative setting. It typically lasts for a few weeks. It is usually incomplete and responds quickly and completely to prednisone.
Tinnitus
Preexisting tinnitus will usually resolve in patients after stapes surgery. However, some patients will complain of new-onset tinnitus. As discussed previously,tinnitus is possibly related to serous labyrinthitis and may improve as the ear continues to heal. When tinnitus persists, patients are treated with reassurance and routine tinnitus measures.
Taste Disturbance
Taste disturbance occurs in approximately 9% of patients. Stretching of the chorda tympani is usually the cause of this rather than nerve sectioning. Therefore, if the nerve has been stretched or otherwise injured, it is preferable to section it. Usually taste disturbance resolves in 3 to 4 months.
Tympanic Membrane Perforation
A small marginal perforation may be repaired by freshening the edges and applying a paper patch. If the perforation has not healed, the process is repeated. If this still fails, a myringoplasty can be performed.
Perilymph Fistula
Perilymph fistula is a rare complication after stapes surgery with an incidence ranging from 3 to 10%. With the use of a small fenestra technique, fistula is rarely seen as a cause of failure in stapes surgery. The patient presents with a mixed conductive sensorineural hearing loss with some vague unsteadiness and, rarely, vertigo. In order to repair this, the prosthesis is carefully removed, a tissue seal is placed over the open oval window, and the prosthesis is replaced. A laser is helpful to remove granulation and scar tissue.
Special Considerations
Ménière’s Disease
Endolymphatic hydrops may occur due to otosclerosis or as two separate disease entities. Stapedectomy in the presence of uncontrolled Ménière’s disease can potentially result in a dead ear and should be avoided. A review of patients with otosclerosis and Ménière’s disease at the House Ear Clinic revealed that stapedectomy does not increase the risk of sensorineural hearing loss for patients with bone conduction thresholds of 35 dB or better at 500 Hz and no high-tone hearing loss. Stapedectomy is contraindicated for patients with levels of 45 dB or greater at 500 Hz and with high-tone loss. In this later group of patients, postmortem histopathologic analysis revealed contact of the saccular membrane or Reissner’s membrane with the stapes footplate, which increased the risk of significant postoperative sensorineural hearing loss.
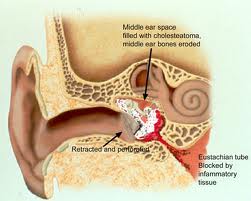
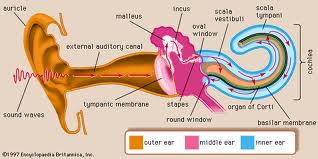
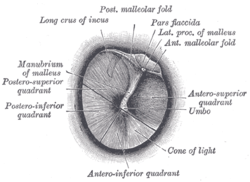
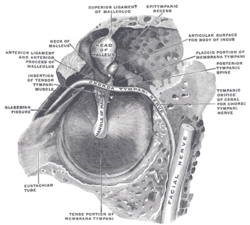
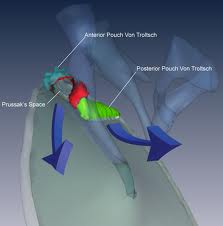
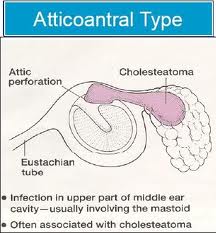
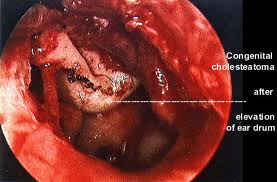
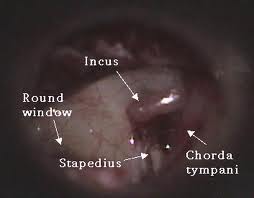
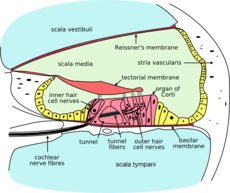
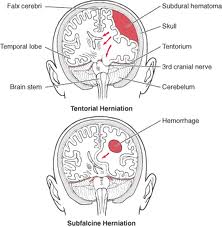
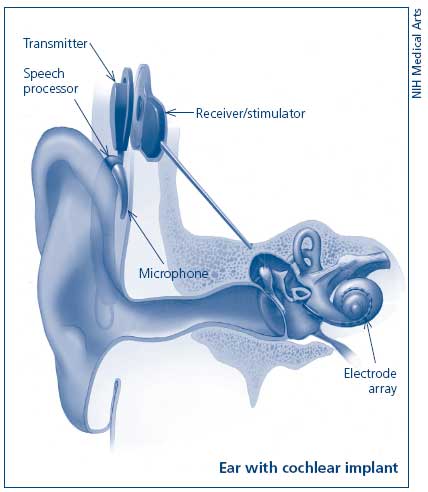
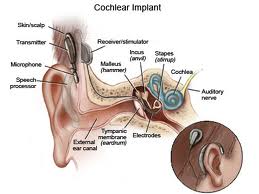
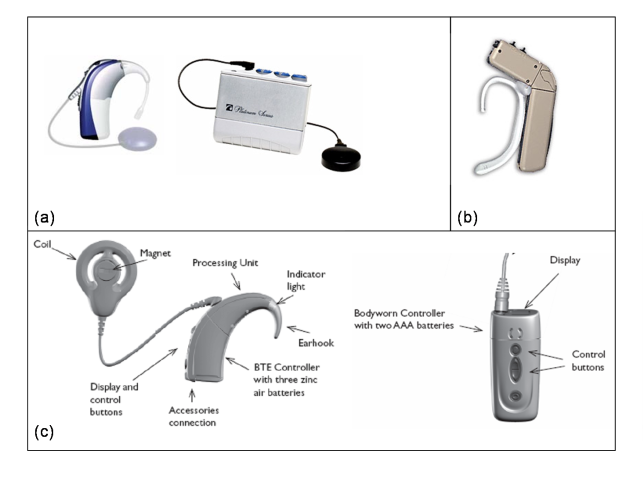

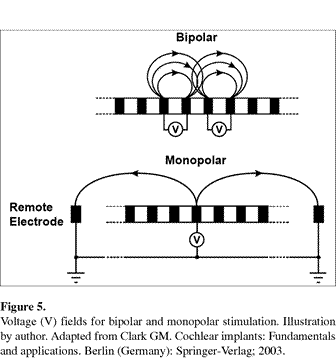
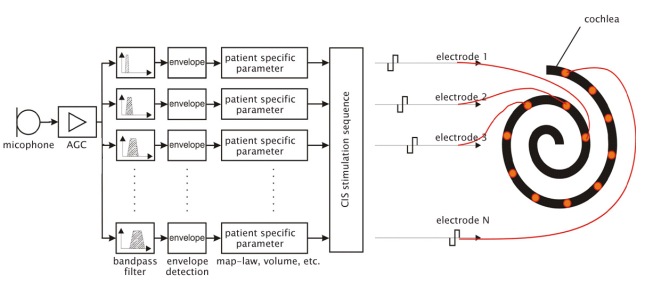
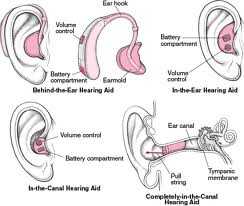
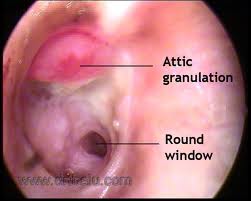
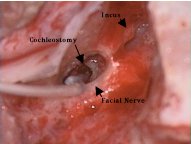

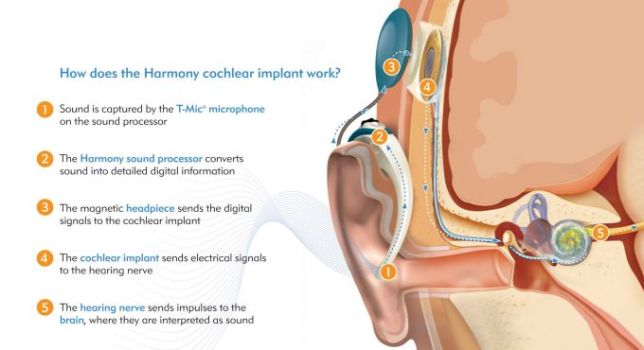
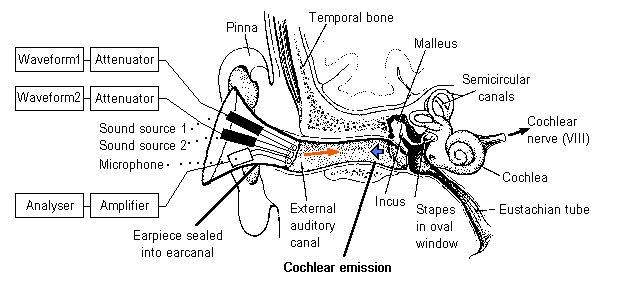
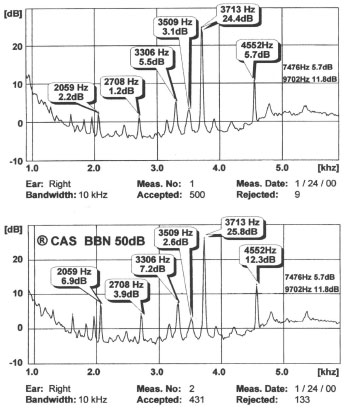

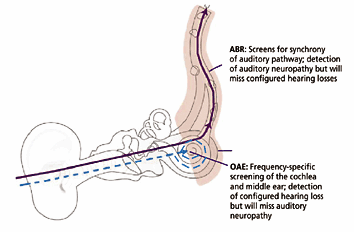
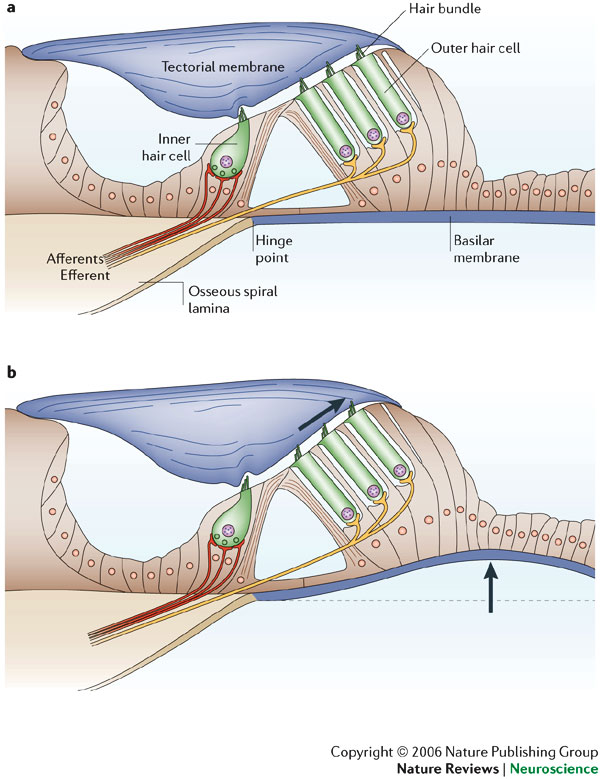
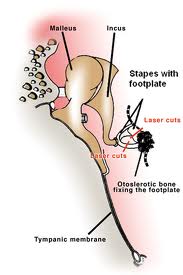
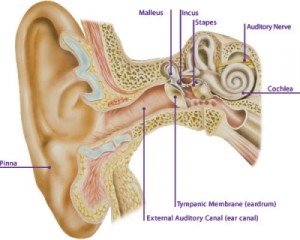
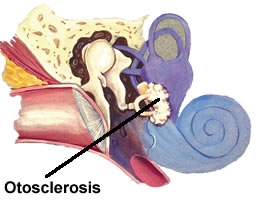
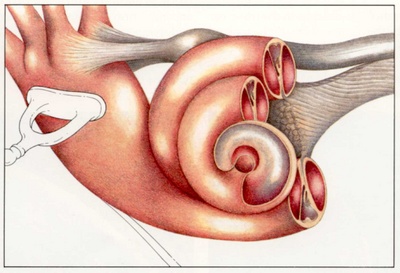
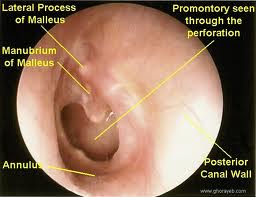

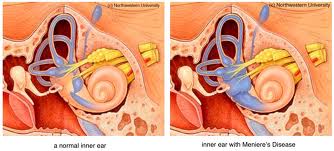
steven
October 9, 2011 at 4:08 pm
excellent articles very helpful for exams.