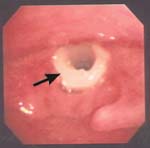
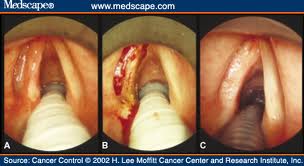
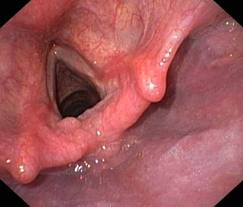
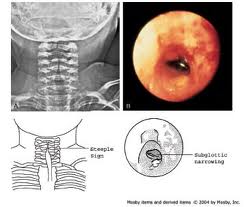
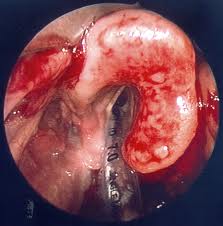
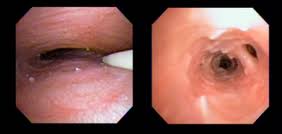
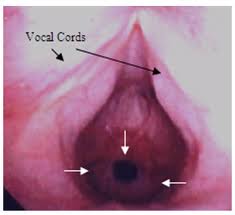
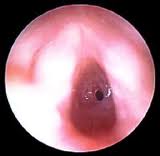
PHARYNX, & LARYNX
Conservation Laryngeal Surgery
Anatomy
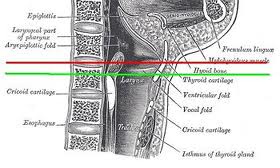
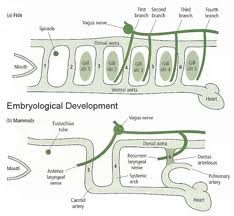
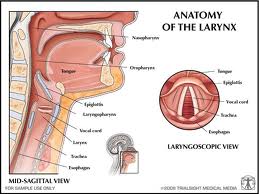
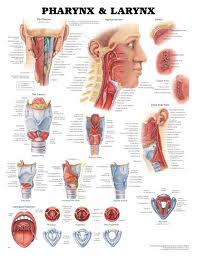
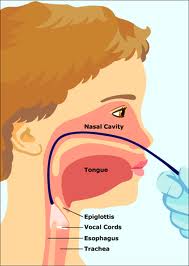
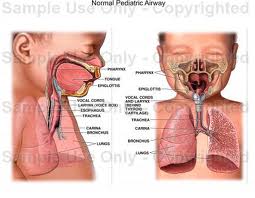
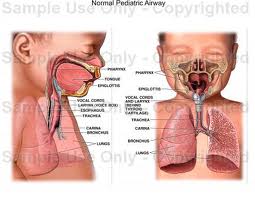
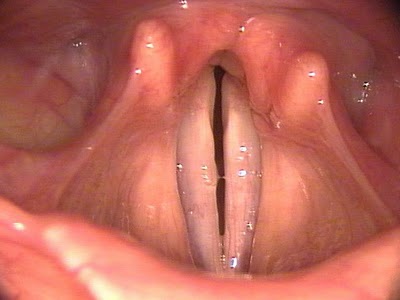
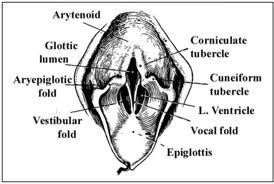
Lymphatic drainage of the larynx is sparse anteriorly and at the level of the glottis. The lymphatic drainage is richer in the supraglottic and subglottic regions, as well as the posterior ½ of the larynx. Lesions above the level of the true vocal cords drain superiorly, while glottic and subglottic lesions drain inferiorly.
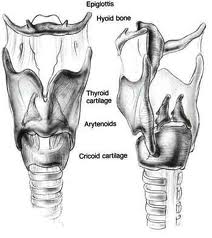
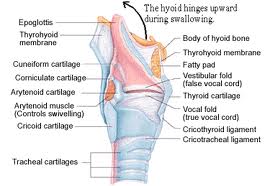
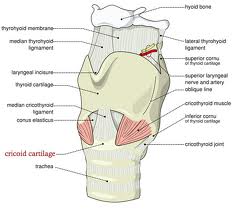
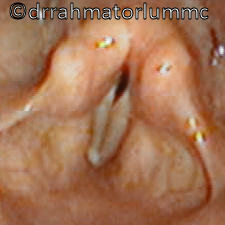
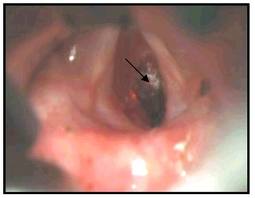
Laryngeal cancer arises from the TVCs approximately 75% of the time. Three fibroelastic membranes serve as the major barriers to the spread of cancer from (and to) the glottic region: the conus elasticus inferiorly, the quadrangular membrane laterally, and the thyrohyoid membrane superiorly. Broyles’ Tendon is the insertion of the vocalis tendon into the thyroid cartilage in the area of the anterior commissure. This is significant because thyroid cartilage perichondrium is deficient in this area, making it a weak point for the spread of malignancy into the thyroid cartilage and on to the extralaryngeal soft tissues of the neck.
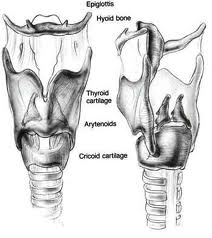
The cricoarytenoid unit consists of the arytenoid cartilage, cricoid cartilage, associated musculature, and the superior laryngeal nerve and recurrent laryngeal nerve for that unit. Of note, a cricoarytenoid unit may retain its function despite compromise of the vocal process or superior aspect of the arytenoid, as long as the body of the arytenoid is preserved.
Pathophysiology of Laryngeal Cancer
Limitation of true vocal cord mobility correlates with a worsening prognosis, especially if the lesion displays an invasive pattern of growth rather than an exophytic or verrucous one.
Kirchner described two types of carcinomatous involvement of the anterior commissure: early lesions that are not invasive and confined to the level of the glottis, and those lesions that invade aggressively and spread superiorly to involve the base of the epiglottis. The latter tend to advance within the cancellous framework of the thyroid cartilage deep to normal appearing soft tissue and imply a poorer prognosis.
Approximately ¼ of early glottic cancer extends to the anterior commissure. Approximately 1/5 of early glottic cancer extends 5 mm or more below the level of the true vocal cords. Likewise, 1/5 extends to involve the supraglottic region.
Early glottic cancer infrequently metastasizes, and when it does, it is almost always to the ipsilateral neck. Lesions limited to the true vocal cords (e.g., T1 and T2) demonstrate a 5% incidence of cervical metastasis, while this figure jumps to 30-40% for T3 lesions.
Approximately 95% of glottic neoplasms are squamous cell carcinoma. Tumor spread is usually superficial and well visualized. Skip lesions, like those seen in the hypopharynx, are rare. Supraglottic squamous cell carcinoma is a different disease process from its glottic counterpart. Supraglottic carcinoma exhibits a much higher incidence of occult nodal metastasis and frank nodal metastasis at presentation. Furthermore, 19% of survivors will develop a second respiratory tract primary within 5 years.
Supraglottic lesions tend to take a long time to spread to the glottis and paraglottic space. However, epiglottic carcinoma demonstrates a predilection for preepiglottic space involvement. When preepiglottic and paraglottic space involvement occurs, it usually involves a broad, pushing front with a pseudocapsule. This pseudocapsule likely arises from the epiglottic perichondrium and the quadrangular membrane.
Early suprahyoid epiglottic lesions are unique in that they rarely invade the preepiglottic space and rarely result in cervical metastasis unless there is occult tongue base involvement.
Special Cases of Glottic Carcinoma
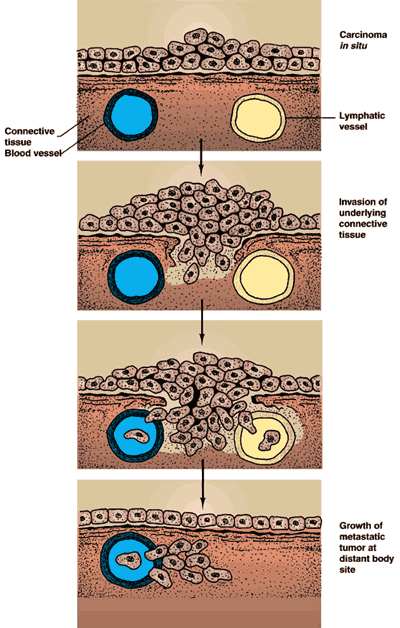
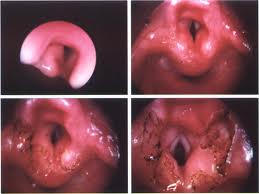
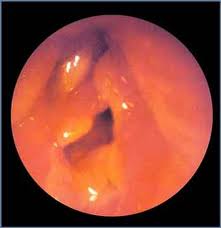
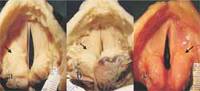
Carcinoma in Situ (CIS)
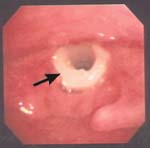
Approximately 1/6 of CIS eventually progresses to invasive carcinoma when treatment is limited to a single excisional biopsy. Radiation therapy is a treatment option, but more failures are associated with radiation as opposed to surgical management in CIS. The classic teaching involves microsuspension laryngoscopy with stripping of the involved TVC epithelium to ensure adequate margins and facilitate histopathologic analysis (to rule out microinvasive carcinoma). A closely monitored program of followup is indicated (e.g., every 2-3 months for 5 years).
Microinvasive Carcinoma
In this case, cancer cells pass through the epithelial basement membrane but do not invade the vocalis muscle. Appropriate treatment entails either a slightly deeper plane of dissection (i.e., taking partial thickness vocalis muscle/ligament with the specimen) or serial epithelial excision Q2-3 months until 2 consecutive specimens without tumor cells are noted. The major difference between microinvasive carcinoma and CIS is that microinvasive carcinoma has metastatic potential, whereas CIS does not.
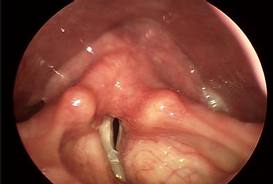
Verrucous Carcinoma
These lesions are characterized by a rough, shaggy surface with rounded, pushing margins. These lesions do not metastasize. Compared to other types of SCCA, these tumors are relatively radioresistant. The treatment of choice is endoscopic resection for smaller lesions, and partial laryngectomy for larger lesions.
Endoscopic Management of Glottic Lesions
For CIS, T1a, and T1b glottic carcinoma, there are essentially three treatment options: conservation surgery, radiation therapy, and microendoscopic CO2 laser excision. The cure rates for all three of these options are approximately equal. Regardless of treatment modality (laser excision versus XRT), local control is approximately 94% for T1a lesions, 71% for T1b lesions, and 83% for T2 lesions. This indicates that anterior commissure involvement (e.g., T1b lesions) portends a worse prognosis for laryngeal conservation regardless of treatment modality. In the US, vertical partial laryngectomy is favored over the laser or XRT for T2 lesions involving the anterior commissure or arytenoid. Tumor features that predict a poor response to XRT and favor use of the laser include increased tumor bulk and overexpression of P53. One tumor factor that predicts a poor result with laser excision is a history of previous XRT.
There are essentially 3 minimally invasive surgical treatment options for early glottic cancer: cold instrumentation, powered instrumentation, and transoral laser excision.
Strong and Jako in 1972 introduced CO2 laser excision for the treatment of laryngeal disease. The advantages they noted were precise control, minimal bleeding, and the absence of post-operative edema.
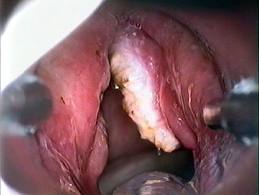
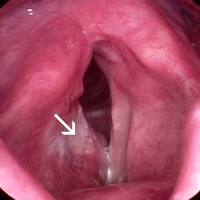
Preoperatively, all patients should undergo a thorough physical examination, including flexible laryngoscopy and videostroboscopy. It is vitally important to assess for the presence or absence of a mucosal wave, which implies the absence or presence of involvement of the vocalis muscle. However, injection of 1:10,000 epinephrine pre-excision has proven more reliable than videostrobe at determining the presence of invasion of the vocal ligament. In addition, any impairment of vibratory patterns of the TVC suggests that a submucosal cordectomy, alone, will not be adequate. Gallo is even more aggressive. She recommends a complete cordectomy for involvement of the anterior commissure, any lesion that infiltrates into the vocal fold, and tumor size >0.7 mm.
Manual pressure applied by an assistant or silk tape over the neck is often useful to improve visualization, especially at the anterior commissure. Microcups should be used to grasp the lesion, and tension applied. The excision should then be performed with solitary laser bursts. Once the cordectomy specimen has been excised, it should be oriented and then sent to surgical pathology for frozen section. If a positive margin is noted, the resection can be extended until healthy margins are obtained. Currently a “safe” margin for CIS or T1 lesions is considered 2-5 mm of surrounding healthy tissue.
Exclusion criteria should be stricter for endoscopic resection of glottic lesions as compared to open conservation laryngeal surgery. Exclusion criteria include deep involvement or fixation of tumor at the anterior commissure, vocal process involvement, involvement of the ventricle (some debate), and subglottic extension (some debate). In the area of the anterior commissure, resection must include thyroid cartilage because of the absence of perichondrium in this region. In addition, endoscopic resection is only appropriate when close followup is possible and appropriate adjuvant therapy is provided when indicated.
Many authors also regard impaired TVC mobility as a contraindication to use of the laser; in a series by Steiner, 11 patients with T2b lesions who received laser excision and post-op XRT had a 5-year disease free survival of 67%. However, the University of Utah introduced a technique that may significantly improve outcomes for T2b lesions. In their series of 11 patients with T2b lesions, they improved the 5-year disease free survival rate to 91% by performing excision of the ipsilateral aryepiglottic fold and hemiepiglottis before excising the glottic specimen. This had the effect of “uncapping” the posterolateral paraglottic space and allowing full exposure of the medial wall of the pyriform sinus and thyroid cartilage from above. This is merely an extension of the concept that adequate visualization of the tumor during endoscopic excision is vital.
In Moreau’s study of 124 patients with glottic lesions, granuloma formation at the anterior commissure was a common occurrence. These granulomas tended to last for several months before spontaneous resolution. Other complications, which were few, included laryngeal hemorrhage, pneumothorax, aspiration pneumonia, subcutaneous air, and prelaryngeal abscess. In addition, several webs resulted from anterior commissure resection; these were treated with repeat endoscopic procedures.
Most European authors advocate the CO2 laser equivalent of vocal cord “stripping” for CIS and microinvasive carcinoma. For CIS, a submucosal cordectomy is advocated; the plane of dissection is the superficial layer of the lamina propria. For microinvasive carcinoma, a subligamental or transmuscular cordectomy is advocated; the plane of dissection is either between the vocal ligament and the vocalis muscle, or through the vocalis muscle (the key point being that at least some vocalis muscle is left intact throughout the full thickness of the cord). They grant this may result in “overtreatment” of many lesions, but this results in excellent oncologic results while maintaining good voice outcomes. In addition, CIS can be very difficult to distinguish from microinvasive carcinoma, especially based upon a small biopsy of mucosal tissue. (Of note, immunohistological staining for Epidermal Growth Factor receptors can help in distinguishing moderate from severe dysplasia.) Most American authors feel that cold instrumentation, alone, is adequate for a plane of dissection superficial to the vocal ligament. The CO2 laser should be used for any transmuscular dissection.
Cordectomies performed in a submucosal or transmuscular plane can be performed safely as a day surgery procedure. Outcomes have been excellent following these procedures. In Damm’s series of 29 patients, local control was achieved in 86% of patients following the first endoscopic procedure; 3 of the 4 patients with recurrence were treated successfully with 1 additional laser procedure, while the other required 4 additional endoscopic procedures. No one required an open procedure for control. In Gallo’s series of 156 laser cordectomy patients with Tcis-T1b lesions, local control was 100% with laser alone at 3 years for CIS. For T1A lesions, 6% experienced local recurrence; all were treated with either XRT or subtotal laryngectomy (no total laryngectomies were required). For T1b lesions, only 9% experienced local recurrence; again, no one required a total laryngectomy for salvage. Flint’s review of the literature suggested a local control rate of 40-100% for glottic Tcis-T2 lesions using the laser.
More extensive procedures should be followed by at least 24-hour observation for airway and swallow safety.
Powered instrumentation deserves mention, though its use in the endoscopic management of laryngeal neoplasms is limited. Flint recommends setting the microdebrider to oscillate at 500 RPM for mucosal lesions on or near the TVC, or 3,000 RPM for larger lesions. The 3.5 mm round window laryngeal blade should be used for superficial lesions, while the 4 mm tricut blade is better for larger lesions. A tissue trap should be used to catch the specimen, which may then be processed and evaluated as a tissue block.
Advantages of powered instrumentation as compared to the laser include improved access to the anterior commissure and subglottis, and elimination of the risk of surgical fire/thermal injury. However, disadvantages include no margin control, and the specimen cannot be oriented. This, of course, is a major drawback. The microdebrider is excellent for the treatment of benign laryngeal lesions. In the setting of malignancy, powered instrumentation may be most useful in terms of “debulking” large, exophytic laryngeal lesions prior to excision with margin control, or to temporarily improve a patient’s airway and avoid tracheotomy prior to more definitive treatment.
Endoscopic Management of Supraglottic Lesions
Unlike glottic carcinoma, surgery is usually favored in the treatment of supraglottic squamous cell carcinoma unless patient factors preclude surgery.
The major contraindications to any form of supraglottic laryngectomy include
- Involvement at the glottic level (Kirchner demonstrated that extension into the infrapetiole/anterior commissure region frequently results in thyroid cartilage invasion)
- Invasion of the cricoid or thyroid cartilage
- Involvement of the tongue base to within 1 cm of the circumvallate papillae.
Vaughan first described the CO2 laser for use in supraglottic squamous cell carcinoma in 1978. Since that time, application of the laser to supraglottic cancer has gained wide acceptance in Europe, but not so in the United States. Some reasons for this may be that the endoscopic approach involves an entirely different treatment paradigm with which most American surgeons are not familiar. In addition, larger lesions are technically more difficult to resect with the laser. And, finally, there has been a proliferation of non-surgical organ preservation protocols in our country.
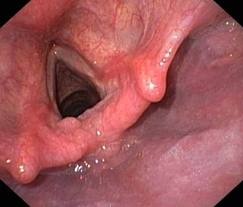
The key to use of the laser in the supraglottic region is optimizing exposure. A bigger area of exposure is required than for glottal surgery. Steiner started to use a bivalved laryngopharyngoscope in the 1980’s. Zeitels later modified this while maintaining the bivalved design to develop the presently popular supraglottiscope.
Positioning works hand-in-hand with the scope to maximize exposure. The Boyce-Jackson position is optimal: extension occurs at the occipitoatlantic joint with the neck flexed on the chest.
Transoral laser resection is most successful when supraglottic lesions are selected for small size and endoscopic accessibility. The supraglottic lesions most amenable to laser resection are those that rest perpendicular to the distal lumen of the supraglottiscope and therefore minimize tangential cutting. These include
- Suprahyoid epiglottic lesions
- Lesions of the aryepiglottic fold
- Lesions of the false vocal fold
Lesions of the infrahyoid epiglottis and upper FVC are more difficult to resect.
Following resection of the specimen, margin analysis is best accomplished by sending the entire specimen for evaluation. Decisions regarding radiation therapy and management of the necks should be based on the pathology of the primary lesion. Fears that this may result in undesirable treatment delay of the necks should be alleviated by the fact that laser resection can be performed as an excisional biopsy at the time of staging endoscopy.
In Zeitels series of 19 patients with T1 and T2N0 supraglottic lesions limited to sites 1-3 above, none of them failed in the neck, no patient required artificial airway intervention, and most patients returned to a normal diet within several days.
Larger lesions in N0 patients are better served post-excision by full-course XRT to the primary and bilateral necks, and this represents a more aggressive form of treatment than XRT alone, particularly in those patients who may not be good candidates for open surgery. Even in these cases, clear margins are usually obtained at the time of laser excision because of the tendency of supraglottic carcinoma to develop a pseudocapsule. In Zeitels series of 23 patients with T2 or T3N0 lesions treated with laser excision and XRT, 16/23 had clear margins at the primary site; none of these patients failed locally. However, of the 7 patients without clear margins, 4 experienced local failure requiring salvage total laryngectomy, and another failed in the neck. In general, completely excising the primary lesion prior to XRT is thought to result in a 20-35% treatment advantage over XRT alone. Though Steiner has used single-modality endoscopic treatment for T2 and T3 lesions, most surgeons advocate post-operative XRT because it is extremely difficult to guarantee comprehensive excision of the preepiglottic and paraglottic space.
Complications related to the use of the CO2 laser in the supraglottis are exceptionally rare. The epiglottis is thought to be a vestigial organ in humans, so swallowing should not be significantly compromised without it. Laryngeal protection is impaired for several days to 6 weeks depending on the extent of laser resection. Patients with a normal preoperative swallow do not experience permanent swallowing deficits with laser resection. Preexisting swallowing impairment such as may be seen in stroke or previous head and neck surgery is a relative contraindication to any partial laryngeal resection. In short, some, but not the majority of patients, may require temporary feeding tubes. This is so when using the laser because the superior laryngeal nerves are not disturbed proximal to the larynx, laryngeal elevation is not impaired by a tracheotomy or disturbance of the suprahyoid musculature, and healing by secondary fibrosis and epithelialization results in a favorable cicatrisation that produces a new supraglottic valve.
Of note, patients who are poor candidates for supraglottic laryngectomy because of pulmonary status are still good candidates for transoral resection. In Zeitels’ study, none of the patients predicted as poor candidates for open supraglottic laryngectomy developed post-operative pulmonary complications.
Hospitalization is usually required for 1-3 days postoperatively. Close involvement with a speech pathologist is very important.
All of this being said, open supraglottic laryngectomy remains the standard surgical management for early supraglottic carcinoma
Vertical Partial Laryngectomy (VPL)
Prior to conservation laryngeal surgery, the primary tumor must be carefully mapped at the time of endoscopy, and it is a good idea to perform a direct laryngoscopy at the time of operation as well, particularly if more than a week has passed since the original endoscopy. Cricoarytenoid joint involvement is a contraindication to any organ preservation surgery; this may be assessed even before direct laryngoscopy by having the patient cough during flexible nasolaryngoscopy. Alternatively, one may palpate the arytenoids at the time of direct laryngoscopy to check for mobility. The difference between a fixed TVC secondary to paraglottic space involvement and a fixed TVC secondary to cricoarytenoid joint involvement can mean the difference between organ preservation and tracheostoma dependence. In addition, subglottic extension should be assessed carefully at the time of endoscopy. This is best accomplished via apneic technique using 0 and 30-degree rigid endoscopes. Other very important features to note during endoscopy are the tumor’s proximity to the AC and arytenoid, and involvement of the ventricle/undersurface of the false vocal cord.
There are five generally accepted contraindications to VPL:
- A fixed true vocal cord
- Involvement of the posterior commissure
- Invasion of bilateral arytenoids
- Bulky transglottic lesions
- Thyroid cartilage invasion
The upper limits of tumors amenable to VPL include
- Up to 5 mm of contralateral TVC involvement
- Up to 15 mm of subglottic extension anteriorly
- Up to 5 mm of subglottic extension posteriorly
- Lesions extending up to the free edge of the false vocal cord superiorly.
Vocal cord fixation (i.e., apparent T3 lesions) suggests the lesion is unsuitable for VPL. 75% of the time, lesions resulting in a fixed vocal cord will extend through the thyroid or cricoid cartilage and involve cartilage beyond the extent of visible tumor.
With respect to patient selection, cardiopulmonary status is an important factor. Aging and chronic obstructive pulmonary disease increase the risk of postoperative atelectasis and pneumonia. The value of preoperative Pulmonary Function Testing (PFTs) is controversial. Some authors recommend the routine use of PFTs. An FEV1 of less than 50-60% of expected for the patient’s age suggests a high risk of pulmonary-associated complications following conservation surgery. However, in 1988, Chow and colleagues demonstrated that a patient’s ability to walk up two flights of stairs is a better predictor of post-operative complications than PFTs. In addition, a chronic and inefficient cough, and purulent sputum bode poorly for the patient’s ability to tolerate organ preservation surgery.
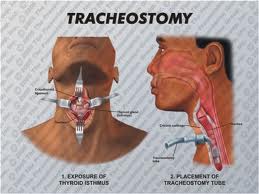
All patients should be evaluated preoperatively by a speech therapist. The patient must also be aware of the need for a temporary feeding tube and tracheostomy.
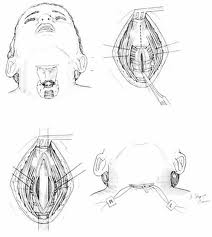
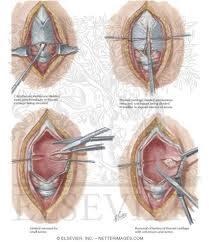
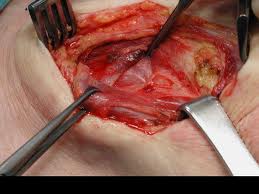
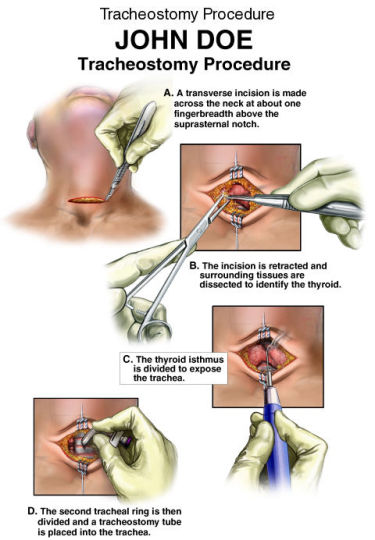
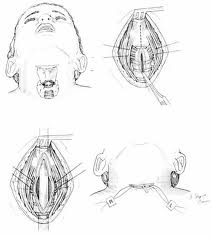
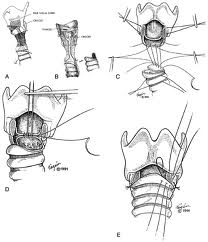
A tracheotomy is required when performing a VPL, and an armored endotracheal tube is placed. An apron incision is then made, and a superior flap elevated in the subplatysmal plane to allow exposure up to the level of the hyoid bone. Care must be taken to avoid injury to the superior laryngeal nerves. After elevation of the flap, cervical lymph nodes should be examined; suspicious nodes should be sent for frozen section. The strap muscles are then divided in the midline. An ipsilateral thyroid lobectomy should be performed if there is >5-10 mm of subglottic involvement. The external thyroid perichondrium is then divided in the midline and along the superior and inferior margins of the thyroid alae. The perichondrium is then elevated to a point parallel with the superior and inferior thyroid cornua. The thyroid cartilage is then divided in the midline, or slightly off midline on the less involved side, with an oscillating saw. The cricothyroid membrane is then cut vertically (or in a transverse direction) and a hemostat is inserted to allow the surgeon to examine the subglottic area directly using a headlight. The internal perichondrium is then elevated off of the deep surface of the thyroid alae. Tufano advocates routinely resecting at least a portion of thyroid cartilage at the glottic level in continuity with the specimen. The tumor is then excised under direct vision with a cuff of normal tissue. The inferior line of excision should extend along the superior margin of the cricoid cartilage. Excessive resection of the epiglottis should be avoided to prevent aspiration. Surgical margins are sent for frozen section analysis.
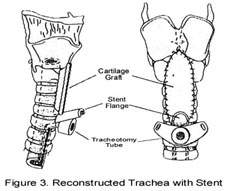
Several options exist for the reconstruction of the surgical defect. With an intact ipsilateral thyroid lamina, these include a skin graft, buccal mucosa, and false vocal cord advancement. When all or part of the ipsilateral thyroid lamina is removed, these include a composite septal cartilage/perichondrial free graft, and an inferiorly and laterally rotated epiglottis. In either case, a bipedicled strap muscle flap is an excellent option; one must anticipate approximately 30% muscle atrophy when insetting the flap. The sternohyoid, sternothyroid, and thyrohyoid muscles may be used and transposed deep to the remaining thyroid ala; the preserved external perichondrium is then used to line the laryngeal lumen.
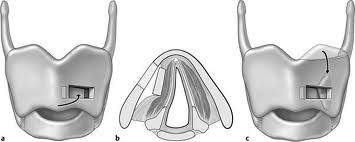
The resection specimen should include the lower ½ of the FVC and all of the TVC (including the arytenoid when necessary). A VPL may be extended to include the entire endolaryngeal circumference except for 1 arytenoid unit and the posterior commissure. In such a case, reconstruction would require bilateral bipedicled muscle flaps. If both sides of the endolarynx undergo excision or reconstruction, then an anterior commissure keel should be used during the healing phase (at least 3 weeks) prior to removal. The keel can be fashioned with anterior limbs that can be tacked down to the soft tissues of the neck with suture.
If the anterior commissure is involved, a central segment of thyroid cartilage may be isolated and removed en bloc with the specimen. In such a case, two longitudinal paramedian incisions would be required when opening the thyroid cartilage rather than a single median thyrotomy. Two major options exist for reconstruction of the anterior commissure: first, the epiglottic petiole may be dissected free and pulled inferiorly into the surgical defect and sutured into place; second, bilateral omohyoid muscle flaps may be used to reconstruct the defect. If the epiglottis is not required for closure, Osguthorpe advocates tacking the petiole to the hyoid in the midline to minimize epiglottic retroversion.
Yet another variant of VPL is imbrication laryngectomy. In this procedure, a through and through excision of a horizontal segment of larynx is performed in continuity with the specimen. The caudal and cephalic portions of the cut thyroid ala are then brought together and overlapped, and the endolaryngeal mucosa is approximated over the cartilage. Thus, the cartilaginous support and soft tissue planes of the larynx are preserved, with the FVC essentially acting as the neocord.
Drains should be placed in the neck intraoperatively. Oral feeds should begin by the end of the first post-operative week. Decannulation should be performed at 1 to 2 weeks postoperatively. Patients can usually resume functional phonation by approximately 4 weeks postoperatively.
- VPL is indicated for T1 or T2 glottic lesions with or without supraglottic extension. At our institution, XRT has been the mainstay as initial therapy for T1 and T2 glottic lesions. However, patients with T1 lesions extending to the anterior commissure demonstrate longer survival and fewer total laryngectomies for salvage when VPL is used rather than XRT as initial therapy. In addition, obese patients with early glottic cancer demonstrate high complication and failure rates following primary XRT. Other factors favoring VPL as opposed to XRT include
- Radioresistant tumors (such as verrucous carcinoma)
- Salivary gland malignancies
- Benign laryngeal tumors
- Patients deemed unreliable for 6 weeks of XRT
- Young patients (due to the theoretical increased risk of late radiation-induced sarcoma)
- Neck nodes >2 cm in size favors primary surgery for both neck and primary.
Furthermore, VPL is considered a safe option in selected cases of tumor recurrence following XRT. Criteria for this include
- The lesion must be limited to one TVC (anterior commissure involvement is acceptable)
- The body of the arytenoid must be free of tumor
- Subglottic extension must be <5-10 mm
- The TVC must be mobile
- Thyroid cartilage must NOT be invaded
- The entire area of pre-XRT tumor involvement must be encompassed in the resection
- The lesion must extend no higher than the lateral wall of the ventricle
The overall 5-year local control rate for VPL is 89-100%. Local control is worse when the AC is involved. The most common site of recurrence for a primary involving the AC is in the subglottis. For T2 lesions, failure rates typically exceed 14%. For T3 lesions, failure rates are highly variable, but typically exceed 30%, likely because most versions of VPL do not fully address the paraglottic space or cricoarytenoid joint. VPL is best suited for T1 lesions without AC involvement, and should be used with caution in the setting of extensive T2 lesions or AC involvement. VPL is best avoided for T3 lesions. When comparing VPL to XRT, outcomes are essentially the same except for T2b lesions, in which case 5-year disease free survival is 64-76% for XRT vs. 73-90% for VPL.
Foremost, early complications following VPL include those complications typically associated with tracheotomy (e.g., tube occlusion, hemorrhage, tracheocutaneous fistula, etc.). Infection, including wound infection and chondritis, is also a possibility. Aspiration and dysphonia are common difficulties following VPL, but these should not persist beyond 3 postoperative weeks.
Late complications include aspiration, chondritis, laryngeal stenosis, severe hoarseness, granulation tissue, and tumor recurrence. Two common causes of local failure include an inability to recognize the inferior margin of the tumor (essential during direct laryngoscopy), and missing spread of cancer outward through the cricothyroid membrane. Granulation and webs may be treated with the CO2 laser and the temporary placement of a keel at the AC. Delayed decannulation can occur in up to 5% of patients; again, the CO2 laser may be helpful. However, when laryngeal stenosis occurs, local recurrence must be ruled out. Aspiration may be managed with injection laryngoplasty. This is best performed within the first 6-8 postoperative weeks.
Voice
Voice is a major factor in comparing treatment modalities with very similar rates of local control. Ultimate voice preservation is slightly more successful following primary treatment with conservation laryngeal surgery than it is following primary XRT (97% vs. 90% local control; Bron 2001). Factors with the biggest impact on voice following XRT are a history of prior TVC “stripping” and smoking after treatment.
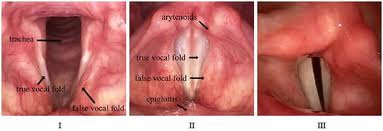
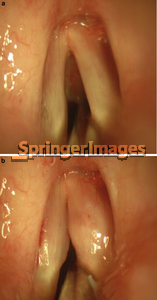
In conservation surgery for glottic lesions, the presence of even a minimal mucosal wave through a lesion noted preoperatively strongly suggests that the lesion is superficial and amenable to microflap or superficial laser excision with a high probability of attaining normal or near normal speech after healing. This fact emphasizes the importance of a pre-treatment videostrobe.
Critical factors in maximizing postoperative voice, particularly in the setting of CIS or microinvasive carcinoma, are limiting the amount of mucosa resected, restoring and maintaining a straight vocal fold edge, and preserving the vocal ligament. To achieve these three aims while still taking adequate surgical margins is actually best achieved using cold microsurgical techniques. However, in Mahieu’s series, 12 of 16 patients with CIS or microinvasive carcinoma had a “normal” voice following CO2 laser excision, and 11 of 16 had a normal post-op videostrobe. This demonstrates that excellent voice outcomes may be achieved using the laser as well.
Following XRT, Stoicheff showed that most patients demonstrate a gradual improvement in voice that plateaus at approximately 4 months post-XRT in >80% of patients, while some patients took up to 2 years to achieve their maximal voice improvement. Lehman demonstrated that, following XRT for glottic carcinoma, videostrobe demonstrated that all patients had reduced or absent mucosal waves bilaterally, and 50% showed irregular glottic closure. Radiation results in fibrosis and decreased mass of the vocal folds. In summary, the literature regarding XRT and voice suggests that most patients are pleased with their voices following XRT, and voice tends to improve gradually following XRT, but the post-irradiation voice is not perfect, and many patients experience persistent vocal problems substantiated by objective data.
Immediately following laser excision for glottic lesions, the voice tends to be breathy and rough. Healing of the TVCs progresses from granulation tissue to the formation of scar tissue with overlying mucosal cover. Recent data suggests that the mucosal wave will be preserved approximately 33% of the time.
In the 5 major studies comparing voice following laser cordectomy to voice following XRT, 3 of 5 showed no significant difference. 2 of 5 showed that the post-irradiated voice was better. Subjective comparisons between the 2 modalities are generally equivalent. The more vocalis muscle resected, the worse the voice.
Total cordectomy results in moderate to severe breathy dysphonia with decreased volume, decreased maximum phonation time, and increased vocal fatigue. Videostrobe demonstrates a severely reduced or absent mucosal wave and incomplete glottic closure. In this setting, the surgical voice is generally thought to be inferior to the post-XRT voice.
With respect to quality of voice following VPL, preservation of functional arytenoid units is the key. Following VPL with strap muscle flap reconstruction, Hirano demonstrated the patients had a rough, breathy, and strained voice with decreased maximum phonation time, increased airflow, and a decreased ability to vary pitch and volume. 80% demonstrated incomplete glottic closure and >50% demonstrated supraglottic hyperfunction. The major finding was the unpredictability of voice results following VPL; post-operative voice varied widely. To date, there have been no studies comparing VPL to XRT with respect to voice.
Discussion
Open conservation laryngeal surgery is associated with a local recurrence rate of approximately 5%. Open procedures are thought to result in slightly higher rates of local control, but these procedures typically require tracheotomy. Following radiation therapy, the recurrence rate of early glottic cancer is higher: from 10% for T1 lesions up to 30% for T2b lesions. When comparing primary surgery to XRT, the treatment of recurrence following XRT is much less satisfactory and frequently requires a total laryngectomy. The primary advantage of laser excision is the curability of recurrence following
Laser Surgery for Laryngeal Cancer
Anatomy
Lymphatic drainage of the larynx is sparse anteriorly and at the level of the glottis. The lymphatic drainage is richer in the supraglottic and subglottic regions, as well as the posterior ½ of the larynx. Lesions above the level of the true vocal cords drain superiorly, while glottic and subglottic lesions drain inferiorly.
Laryngeal cancer arises from the TVCs approximately 75% of the time. Three fibroelastic membranes serve as the major barriers to the spread of cancer from (and to) the glottic region: the conus elasticus inferiorly, the quadrangular membrane laterally, and the thyrohyoid membrane superiorly. Broyles’ Tendon is the insertion of the vocalis tendon into the thyroid cartilage in the area of the anterior commissure. This is significant because thyroid cartilage perichondrium is deficient in this area, making it a weak point for the spread of malignancy into the thyroid cartilage and on to the extralaryngeal soft tissues of the neck.
The cricoarytenoid unit consists of the arytenoid cartilage, cricoid cartilage, associated musculature, and the superior laryngeal nerve and recurrent laryngeal nerve for that unit. Of note, a cricoarytenoid unit may retain its function despite compromise of the vocal process or superior aspect of the arytenoid, as long as the body of the arytenoid is preserved.
Pathophysiology of Laryngeal Cancer
Limitation of true vocal cord mobility correlates with a worsening prognosis, especially if the lesion displays an invasive pattern of growth rather than an exophytic or verrucous one.
Kirchner described two types of carcinomatous involvement of the anterior commissure: early lesions that are not invasive and confined to the level of the glottis, and those lesions that invade aggressively and spread superiorly to involve the base of the epiglottis. The latter tend to advance within the cancellous framework of the thyroid cartilage deep to normal appearing soft tissue and imply a poorer prognosis.
Approximately ¼ of early glottic cancer extends to the anterior commissure.
Approximately 1/5 of early glottic cancer extends 5 mm or more below the level of the true vocal cords. Likewise, 1/5 extends to involve the supraglottic region.
Early glottic cancer infrequently metastasizes, and when it does, it is almost always to the ipsilateral neck. Lesions limited to the true vocal cords (e.g., T1 and T2) demonstrate a 5% incidence of cervical metastasis, while this figure jumps to 30-40% for T3 lesions.
Approximately 95% of glottic neoplasms are squamous cell carcinoma. Tumor spread is usually superficial and well visualized. Skip lesions, like those seen in the hypopharynx, are rare.
Supraglottic squamous cell carcinoma is a different disease process from its glottic counterpart. Supraglottic carcinoma exhibits a much higher incidence of occult nodal metastasis and frank nodal metastasis at presentation. Furthermore, 19% of survivors will develop a second respiratory tract primary within 5 years.
Supraglottic lesions tend to take a long time to spread to the glottis and paraglottic space. However, epiglottic carcinoma demonstrates a predilection for preepiglottic space involvement. When preepiglottic and paraglottic space involvement occurs, it usually involves a broad, pushing front with a pseudocapsule. This pseudocapsule likely arises from the epiglottic perichondrium and the quadrangular membrane.
Early suprahyoid epiglottic lesions are unique in that they rarely invade the preepiglottic space and rarely result in cervical metastasis unless there is occult tongue base involvement.
Background on Lasers
Laser is an acronym for light amplification by the stimulated emission of radiation. Einstein postulated the theoretical foundation of laser action, stimulated emission of radiation, in 1917. Einstein postulated that the spontaneous emission of electromagnetic radiation from an atomic transition has an enhanced rate in the presence of similar electromagnetic radiation. Maiman built the first laser in 1960. With synthetic ruby crystals, this laser produced electromagnetic radiation at a wavelength of 0.69 μm in the visible range of the spectrum. Although the laser energy produced by Maiman’s ruby laser lasted less than 1 ms, it paved the way for explosive development and widespread application of this technology.
Two important advances allowed the laser to be useful in otolaryngology: (1) in 1965, the carbon dioxide (co2) laser was developed, and (2) in 1968, Polanyi developed the articulated arm to deliver the infrared radiation from the co2 laser to remote targets. He combined his talents with Jako and used the articulated arm and the co2 laser in laryngeal surgery. Simpson and Polanyi described the series of experiments and new instrumentation that made this work possible.
A laser is an electro-optical device that emits organized light (rather than the random-pattern light emitted from a light bulb) in a very narrow intense beam by a process of optical feedback and amplification.
Electrons can change their orbits, thereby changing the energy state of the atom. During excitation, an electron can make the transition from a low-energy level to a higher energy level. Excitation that comes from the electron interacting with light (a photon) is termed absorption. The atom always seeks its lowest energy level (i.e., the ground state).
Therefore, the electron will spontaneously drop from the high-energy level back to the lowest energy level in a very short time (typically 10-8 sec). As the electron spontaneously drops from the higher energy level to the lower energy level, the atom must give up the energy difference. The atom emits the extra energy as a photon of light in a process termed the spontaneous emission of radiation.
All laser devices have an optical resonating chamber (cavity) with two mirrors. The space between these mirrors is filled with an active medium, such as Ar, Nd:YAG, or co2. An external energy source (e.g., an electric current) excites the active medium within the optical cavity. This excitation causes many atoms of the active medium to be raised to a higher energy state. A population inversion occurs when more than half of the atoms in the resonating chamber have reached a particular excited state. Spontaneous emission is taking place in all directions. Light (photons) emitted in the direction of the long axis of the laser is retained within the optical cavity by multiple reflections off of the precisely aligned mirrors. One mirror is completely reflective,
and the other is partially transmissive. Stimulated emission occurs when a photon interacts with an excited atom in the optical cavity. This yields pairs of identical photons that are of equal wavelength, frequency, and energy and are in phase with each other. This process occurs at an increasing rate with each passage of the photons through the active medium.
With most surgical lasers, the physician can control three variables: (1) power (measured in watts); (2) spot size (measured in millimeters); and (3) exposure time (measured in seconds).
Of power, spot size, and exposure time, power is the least useful variable and may be kept constant with widely varying effects, depending on the spot size and the duration of exposure. For example, the relationship between power and depth of tissue injury becomes logarithmic when the power and exposure time are kept constant and the spot size is varied.
Irradiance is a more useful measure of the intensity of the beam at the focal spot than power is because it considers the surface area of the focal spot. Specifically, irradiance is expressed (in W/cm2) as: Irradiance = Power in the focal spot/Area of the focal spot.
Power and spot size are considered together, and a combination is selected to produce the appropriate irradiance. If the exposure time is kept constant, the relationship between irradiance and depth of injury is linear as the spot size is varied. Irradiance is the most important operating parameter of a surgical laser at a given wavelength. Therefore, surgeons should calculate the appropriate irradiance for each procedure to be performed.
These calculations allow the surgeon to control, in a predictable manner, the tissue effects when changing from one focal length to another (e.g., from 400 mm for microlaryngeal surgery to 125 mm for hand-held surgery). Irradiance varies directly with power and inversely with surface area. This relationship of surface area to beam diameter is important when evaluating the power density because the larger the surface area, the lower the irradiance; conversely, the smaller the surface area, the higher the irradiance.
Depth of focus is realized when a camera is focused. With a camera, a range of objects is in focus, which can be set without carefully measuring the distance between the object and the lens. The preceding equations show that a long focal length lens leads to a large beam waist, which also translates as a large depth of focus.
The size of the laser beam on the tissue (spot size) can therefore be varied in two ways: (1) because the minimum beam diameter of the focal spot increases directly with increasing the focal length of the laser focusing lens, the surgeon can change the focal length of the lens to obtain a particular beam diameter. As the focal length decreases, a corresponding decrease occurs in the size of the focal spot. Also, the smaller the spot size is for any given power output, the greater the corresponding power density. (2) The surgeon can also vary the spot size by working in or out of focus. The minimum beam diameter and highest power concentration occur at the focal plane, where much of the precise cutting and vaporization is carried out. As the distance from the focal plane increases, the laser beam diverges or becomes unfocused. The cross-sectional area of the spot increases and thus lowers the power density for a given output. The size of the focal spot depends on the focal length of the laser lens and whether the surgeon is working in or out of focus.
The surgeon can vary the amount of energy delivered to the target tissue by varying the exposure time. Fluence refers to the amount of time (measured in seconds) that a laser beam irradiates a unit area of tissue at a constant irradiance. Fluence is a measure, then, of the total amount of laser energy per unit area of exposed target tissue. Fluence varies directly with the length of the exposure time, which can be varied by working in the pulsed mode (duration, 0.05–0.5 sec) or in the continuous mode.
When electromagnetic energy (incident radiation) interacts with tissue, the tissue reflects, absorbs, transmits, and scatters portions of the light. The surgical interaction of this radiant energy with tissue is caused only by that portion of light that is absorbed (i.e., the incident radiation minus the sum of the reflected and transmitted portions).
The actual tissue effects produced by the radiant energy of a laser vary with the laser’s wavelength. Each type of laser exhibits different characteristic biologic effects on tissue and is therefore useful for different applications. However, certain similarities exist regarding the nature of laser light interaction with biologic tissue. The lasers used in medicine and surgery today can be ultraviolet, meaning the interactions are a complex mixture of heating and photodissociation of chemical bonds. The more commonly used lasers emit light in the visible or the infrared region of the electromagnetic spectrum, and their primary form of interaction with biologic tissue leads to heating. Therefore, if the radiant energy of a laser is to exert its effect on the target tissue, it must be absorbed by the target tissue and converted to heat. Scattering tends to spread the laser energy over a larger surface area of tissue, but it limits the penetration depth. The shorter the wavelength of light, the more it is scattered by the tissue. If the radiant energy is reflected from or transmitted through the tissue, no effect will occur. To select the most appropriate laser system for a particular application, the surgeon should thoroughly understand these characteristics regarding the interaction of laser light with biologic tissue
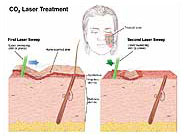
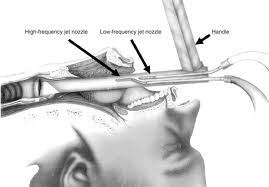
co2 lasers produce light with a wavelength of 10.6 μm in the infrared (invisible) range of the electromagnetic spectrum. A second, built-in, coaxial helium-neon laser is necessary because its red light indicates the site where the invisible co2 laser beam will impact the target tissue. Thus, this laser acts as an aiming beam for the invisible co2 laser beam. The radiant energy produced by the co2 laser is strongly absorbed by pure, homogeneous water and by all biologic tissues high in water content. The extinction length of this wavelength is about 0.03 mm in water and in soft tissue. Reflection and scattering are negligible. Because absorption of the radiant energy produced by the co2 laser is independent of tissue color and because the thermal effects produced by this wavelength on adjacent nontarget tissues are minimal, the co2 laser has become extremely versatile in otolaryngology.
With current technology, light from the co2 laser cannot be transmitted through existing flexible fiberoptic endoscopes, although research and development of a suitable flexible fiber for transmission of this wavelength is being carried out internationally. At present, the radiant energy of this laser is transmitted from the optical resonating chamber to the target tissue via a series of mirrors through an articulating arm to the target tissue. The co2 laser can be used free-hand for macroscopic surgery, attached to the operating microscope for microscopic surgery, and adapted to an endoscopic coupler for bronchoscopic surgery. This latter application requires rigid nonfiberoptic bronchoscopes. Pattern generators coupled with a micromanipulator on the
operating microscope have also been introduced to help with the surgical precision in laryngology.
Laser Safety
The laser is a precise but potentially dangerous surgical instrument that must be used with caution. Although distinct advantages are associated with the use of laser surgery in the management of certain benign and malignant diseases of the upper aerodigestive tract, these advantages must be weighed against the risks of complications. Because of these risks, the surgeon must first determine if the laser offers an advantage over conventional surgical techniques. For the surgeon to use good judgment in the selection and use of lasers in practice, prior experience in laser surgery is necessary. Hospitals that offer laser surgery should appoint a laser safety officer and set up a laser safety committee consisting of the laser safety officer, physicians using the laser, anesthesiologists, operating room nurses, a hospital administrator, and a biomedical engineer. The purpose of this committee is to develop policies and procedures for the safe use of lasers within the hospital
Several structures of the eye are at risk. The area of injury usually depends on which structure absorbs the most radiant energy per volume of tissue. Depending on the wavelength, corneal or retinal burns, or both, are possible from acute exposure to the laser beam. The possibility for corneal or lenticular opacities (cataracts) or retinal injury exists after chronic exposure to excessive levels of laser radiation. Retinal effects occur when the laser emission wavelength occurs in the visible and near-infrared range of the electromagnetic spectrum (0.4–1.4 μm). To reduce the risk of ocular damage during cases involving the laser, certain precautions should be followed. Protecting the eyes of the patient, surgeon, and other operating room personnel must be addressed. The actual protective device will vary according to the wavelength of the laser used. A sign should be placed outside the operating room door warning all persons entering the room to wear protective glasses because the laser is in use. In addition, extra glasses for the specific wavelength in use should be placed on a table immediately outside the room. The doors to the operating room should remain closed during laser use.
Patients undergoing co2 laser surgery of the upper aerodigestive tract should have a double layer of saline-moistened eye pads placed over the eyes. All operating room personnel should wear protective eyeglasses with side protectors. Regular eyeglasses or contact lenses protect only the areas covered by the lens and do not provide protection from possible entry of the laser beam from the side. When working with the operating microscope and the co2 laser, the surgeon need not wear protective glasses. The optics of the microscope provide the necessary protection. When working with the Nd:YAG laser, all operating room personnel (and the patient) must wear wavelength-specific protective eyeglasses that are usually blue-green. Although the beam direction and point of impact may appear to be confined within the endoscope, inadvertent deflection of the beam may occur because of a faulty contact, a break in the fiber, or accidental disconnection between the fiber and endoscope. Special wavelength-specific filters are available for flexible and rigid bronchoscopes. When these filters are in place, the surgeon need not wear protective eyeglasses.
When working with the Ar, KTP, or dye lasers, all personnel in the operating room, including the patient, should again wear wavelength-specific protective eyeglasses that are usually amber. When undergoing photocoagulation for selected cutaneous vascular lesions of the face, the patient usually wears protective metal eye shields rather than protective eyeglasses. Similar precautions are necessary for the visible and near-infrared wavelength lasers. The major difference is the type of eye protection that is worn.
The patient’s exposed skin and mucous membranes outside the surgical field should be protected by a double layer of saline-saturated surgical towels, surgical sponges, or lap pads. When microlaryngeal laser surgery is being performed, the beam might partially reflect off the proximal rim of the laryngoscope rather than go down it. Thus, saline-saturated surgical towels completely drape the patient’s face. Only the proximal lumen of the laryngoscope is exposed. Great care must be exercised to keep the wet draping from drying out. It should occasionally be moistened during the procedure. Teeth in the operative field also need to be protected. Saline-saturated Telfa, surgical sponges, or specially constructed metal dental impression trays can be used. Meticulous attention is paid to the protective draping procedures at the beginning of the surgery. The same attention should be paid to the continued protection of the skin and teeth during the surgical procedure.
Two separate suction setups should be available for all laser cases in the upper aerodigestive tract. One provides for adequate smoke and steam evacuation from the operative field; whereas the second is connected to the surgical suction tip for the aspiration of blood and mucus from the operative wound. When performing laser surgery with a closed anesthetic system, the surgeon should use constant suctioning to remove laser-induced smoke from the operating room. This helps to prevent inhalation by the patient, surgeon, and operating room personnel. When the anesthetic system is open or has jet ventilation systems, suctioning should be intermittent to maintain the forced inspiratory oxygen at a safe level. Laryngoscopes, bronchoscopes, operating platforms, mirrors, and anterior commissure and ventricle retractors with built-in smoke-evacuating channels facilitate the evacuation of smoke from the operative field.
Complications
Aside from a few minor eye injuries from a laser beam exposure, most serious accidental injuries related to laser use can be traced to the ignition of surgical drapes and airway tubes.[41] Because the anesthesiologist is also concerned with the airway and because potent oxidizing gases pass through the airway in close approximation to the path of the laser beam, it is necessary to develop a team approach to the anesthetic management of the patient undergoing laser surgery of the upper aerodigestive tract. It is recommended that anesthesiologists involved with laser surgery cases attend a didactic session devoted to this subject. Finally, the operating room staff must be educated with regard to laser surgery. Attendance at an inservice workshop with exposure to clinical laser biophysics and the basic workings of the laser, as well as hands-on orientation should be the minimal requirement for nurses to participate in laser surgery.
One of the most devastating complications of laser surgery of the aerodigestive tract is endotracheal tube ignition and resulting injury to the laryngotracheal mucosa. At present, a nonflammable, universally accepted endotracheal tube for all types of laser surgery of the upper aerodigestive tract does not exist. The traditional polyvinyl endotracheal tube should not be used, either wrapped or unwrapped. It offers the least resistance to penetration by the laser beam of all
The endotracheal tubes that have been tested, fire-breakdown products are toxic, and tissue destruction associated with combustion of this tube is the most severe. Endotracheal tubes for laser surgery that are wavelength specific are now available from several manufacturers and should be used at all times unless jet ventilation techniques are used.
Protection of the endotracheal tube from direct or reflected laser beam irradiation is of primary importance. If the laser beam strikes an unprotected endotracheal tube carrying oxygen, ignition of the tube could result in a catastrophic, intraluminal, blowtorch-type endotracheal tube fire. Protection should also be provided for the cuff of the endotracheal tube. Methylene blue-colored saline should be used to inflate the cuff. Saline-saturated cottonoids are then placed above the cuff in the subglottic larynx to further protect the cuff. These cottonoids require frequent moistening during the procedure. If the cuff deflates from an errant hit by the laser beam, the already saturated cottonoids turn blue to warn the surgeon of impending danger. The tube should then be removed and replaced with a new one. Use of the microlaryngeal operating platform offers further protection against potential danger. Inserted into the subglottic larynx above the level of the packed cottonoids, this unique instrument serves as a back stop to protect the cottonoids, endotracheal tube, and cuff from any direct or reflected laser beam irradiation.
Complications related to the use of the CO2 laser in the supraglottis are exceptionally rare. The epiglottis is thought to be a vestigial organ in humans, so swallowing should not be significantly compromised without it. Laryngeal protection is impaired for several days to 6 weeks depending on the extent of laser resection. Patients with a normal preoperative swallow do not experience permanent swallowing deficits with laser resection. Preexisting swallowing impairment such as may be seen in stroke or previous head and neck surgery is a relative contraindication to any partial laryngeal resection. In short, some, but not the majority of patients, may require temporary feeding tubes. This is so when using the laser because the superior laryngeal nerves are not disturbed proximal to the larynx, laryngeal elevation is not impaired by a tracheotomy or disturbance of the suprahyoid musculature, and healing by secondary fibrosis and epithelialization results in a favorable cicatrisation that produces a new supraglottic valve.
In Moreau’s study granuloma formation at the anterior commissure was a common occurrence. These granulomas tended to last for several months before spontaneous resolution. Other complications, which were few, included laryngeal hemorrhage, pneumothorax, aspiration pneumonia, subcutaneous air, and prelaryngeal abscess. In addition, several webs resulted from anterior commissure resection; these were treated with repeat endoscopic procedures.
The transoral management of squamous cell carcinoma of the larynx using the co2 laser is an obvious extension of the application of this surgical instrument. The advantages of precision, increased hemostasis, and decreased intraoperative edema allow the surgeon to perform exquisitely accurate and relatively bloodless endoscopic surgery of the larynx.
A comprehensive report on the results of TLM was given by Steiner and his colleagues at the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) in Budapest. His reports were based on 606 patients treated from 1979 to 1986 in Erlangen-Nurnberg or 1986 to 1993 in Göttingen. The last Erlangen entry was in January 1994, and the last Göttingen entry was in December 1995. The only exclusions were patients with simultaneous second primary cancers, thus not treatable for cure. Of the patients, 360 had early glottic cancer, 43 had early supraglottic disease, 147 had late glottic carcinoma, and 56 had late supraglottic cancer. The T distributions were pTis; 45 patients, pT1; 228 patients, pT2; 231 patients, pT3; 69 patients and pT4; 33. As might be expected, the Tis and T1a cases did extremely well and will receive no further comment.
Combining the pTis to pT2a patients, there were 35 recurrent cancers amongst 360 TLMs. Of these 35 recurrences, 5 occurred more than 5 years after initial treatment (thus possibly were second primaries).
Of the 35 patients, 27 were salvaged by functional surgery, mainly by transoral laser micro-re-resection. Eight patients proceeded to laryngectomy. Of the 360 (0.5%), 2 died from the glottic cancer. Six developed neck metastases, 3 with their primary controlled and 3 with recurrent cancer at the primary site. During the course of their follow-up, 23 patients (6.4%) developed second primaries, and 16 (5%) died of their second primary. The commonest cause of death in the whole group was intercurrent disease—64 patients (17.5%). The 5-year Kaplan-Meier survivals were 87% for the “very early” glottic group and 83% for the “early” cases. TLM preserved voice in 352 of the 360 patients (98%), and was judged to be of satisfactory quality in 90%. One patient bled. No one needed a tracheotomy.
Endoscopic Management of Glottic Lesions
For CIS, T1a, and T1b glottic carcinoma, there are essentially three treatment options: conservation surgery, radiation therapy, and microendoscopic CO2 laser excision. The cure rates for all three of these options are approximately equal.
Regardless of treatment modality (laser excision versus XRT), local control is approximately 94% for T1a lesions, 71% for T1b lesions, and 83% for T2 lesions. This indicates that anterior commissure involvement (e.g., T1b lesions) portends a worse prognosis for laryngeal conservation regardless of treatment modality. In the US, vertical partial laryngectomy is favored over the laser or XRT for T2 lesions involving the anterior commissure or arytenoid. Tumor features that predict a poor response to XRT and favor use of the laser include increased tumor bulk and overexpression of P53. One tumor factor that predicts a poor result with laser excision is a history of previous XRT.
There are essentially 3 minimally invasive surgical treatment options for early glottic cancer: cold instrumentation, powered instrumentation, and transoral laser excision.
Strong and Jako in 1972 introduced CO2 laser excision for the treatment of laryngeal disease. The advantages they noted were precise control, minimal bleeding, and the absence of post-operative edema.
Preoperatively, all patients should undergo a thorough physical examination, including flexible laryngoscopy and videostroboscopy. It is vitally important to assess for the presence or absence of a mucosal wave, which implies the absence or presence of involvement of the vocalis muscle. However, injection of 1:10,000 epinephrine pre-excision has proven more reliable than videostrobe at determining the presence of invasion of the vocal ligament. In addition, any impairment of vibratory patterns of the TVC suggests that a submucosal cordectomy, alone, will not be adequate. Gallo is even more aggressive. She recommends a complete cordectomy for involvement of the anterior commissure, any lesion that infiltrates into the vocal fold, and tumor size >0.7 mm.
Manual pressure applied by an assistant or silk tape over the neck is often useful to improve visualization, especially at the anterior commissure. Microcups should be used to grasp the lesion, and tension applied. The excision should then be performed with solitary laser bursts. Once the cordectomy specimen has been excised, it should be oriented and then sent to surgical pathology for frozen section. If a positive margin is noted, the resection can be extended until healthy margins are obtained. Currently a “safe” margin for CIS or T1 lesions is considered 2-5 mm of surrounding healthy tissue.
Exclusion criteria should be stricter for endoscopic resection of glottic lesions as compared to open conservation laryngeal surgery. Exclusion criteria include deep involvement or fixation of tumor at the anterior commissure, vocal process involvement, involvement of the ventricle (some debate), and subglottic extension (some debate). In the area of the anterior commissure, resection must include thyroid cartilage because of the absence of perichondrium in this region. In addition, endoscopic resection is only appropriate when close followup is possible and appropriate adjuvant therapy is provided when indicated.
Many authors also regard impaired TVC mobility as a contraindication to use of the laser; in a series by Steiner, 11 patients with T2b lesions who received laser excision and post-op XRT had a 5-year disease free survival of 67%. However, the University of Utah introduced a technique that may significantly improve outcomes for T2b lesions. In their series of 11 patients with T2b lesions, they improved the 5-year disease free survival rate to 91% by performing excision of the ipsilateral aryepiglottic fold and hemiepiglottis before excising the glottic specimen. This had the effect of “uncapping” the posterolateral paraglottic space and allowing full exposure of the medial wall of the pyriform sinus and thyroid cartilage from above. This is merely an extension of the concept that adequate visualization of the tumor during endoscopic excision is vital.
Moreau performed a retrospective study of 160 patients treated from 1988 to 1996 to determine if laser endoscopic microsurgery is a reliable and appropriate approach in the treatment of laryngeal cancers. Glottic tumors were treated with either type I, type II, or type III cordectomy, with or without conservation of an inferior muscular band, and extended if necessary to all or part of the contralateral cord. For supraglottic cancers, an excision limited to a part of the vestibule, a trans-preepiglottic resection, or a radical supraglottic resection was carried out. They found that corrected actuarial survival at 5 years was 97% for the 98 infiltrative glottic tumors and 100% for the 18 infiltrative supraglottic and 27 in situ carcinomas. No local recurrences were noted, in either the group of 118 infiltrating cancers (in whom two precancerous lesions were treated with a further laser excision), or in the 27 in situ carcinomas. Local control was thus 100%. One patient died of his cancer, with lung metastases after neck recurrence. He concluded that, like Steiner and Rudert, his series demonstrated the oncologic validity of this surgical approach to the treatment of unadvanced glottic tumors.
Gallo et al performed a retrospective study of 151 patients treated from April 1982 to June to define when laser resection of early-stage glottic carcinoma is indicated and to compare the results obtained by laser surgery with other therapeutic options. Glottic tumors were treated with type III, type IV, and type Va cordectomies according to the classification of endoscopic cordectomies proposed by the European Laryngological Society in 2000.
They found that all patients with carcinoma in situ Tis were free of disease with local control rate at 3 years of 100%; 2 died of other causes without evidence of local recurrence with an overall survival rate at 3 years of 83.2%. Of the 117 patients with stage T1a cancer, 110 are free of disease at 3 years with local control rate of 94%; 4 patients died of other causes without evidence of local recurrence with an overall survival rate of 96.5%. Of the 22 patients with stage T1b cancer, 20 are free of disease at 3 years with a local control rate of 91%; 1 patient died of other causes without evidence of local recurrence with an overall survival rate at 3 years of 95.4%. They concluded that endoscopic laser surgery is an efficacious and cost-effective treatment for early stage glottic cancer.
Gallo et al also noted that the removal of 2 to 5 mm of healthy tissue surrounding the neoplastic lesion is the suggested measurements in the current literature. They stated that a dilemma arises when treating a tumor of the vocal cord, which extends to (T1a) or involves (T1b) the anterior commissure. Under these circumstances, the removal of the anterior commissure, together with a variable portion of the contralateral vocal cord, can be helpful in obtaining safety margins. Therefore, they recommended that the transmuscular cordectomy (type III) is indicated in cases of small superficial tumors of the mobile vocal fold (T1a); the total cordectomy (type IV) is indicated in cases of T1a cancer with extension to the anterior commissure, and/or when the tumor involves the vocal fold in an infiltrative pattern and/or when the tumor size is more than 0.7 mm; the extended cordectomy encompassing the contralateral vocal fold (type Va) is indicated in cases of T1b cancer involving the anterior commissure or in horseshoe lesions.
Most European authors advocate the CO2 laser equivalent of vocal cord “stripping” for CIS and microinvasive carcinoma. For CIS, a submucosal cordectomy is advocated; the plane of dissection is the superficial layer of the lamina propria. For microinvasive carcinoma, a subligamental or transmuscular cordectomy is advocated; the plane of dissection is either between the vocal ligament and the vocalis muscle, or through the vocalis muscle (the key point being that at least some vocalis muscle is left intact throughout the full thickness of the cord). They grant this may result in “overtreatment” of many lesions, but this results in excellent oncologic results while maintaining good voice outcomes. In addition, CIS can be very difficult to distinguish from microinvasive carcinoma, especially based upon a small biopsy of mucosal tissue. (Of note, immunohistological staining for Epidermal Growth Factor receptors can help in distinguishing moderate from severe dysplasia.) Most American authors feel that cold instrumentation, alone, is adequate for a plane of dissection superficial to the vocal ligament. The CO2 laser should be used for any transmuscular dissection.
Endoscopic Management of Supraglottic Lesions
Unlike glottic carcinoma, surgery is usually favored in the treatment of supraglottic squamous cell carcinoma unless patient factors preclude surgery.
The major contraindications to any form of supraglottic laryngectomy include
- Involvement at the glottic level (Kirchner demonstrated that extension into the infrapetiole/anterior commissure region frequently results in thyroid cartilage invasion)
- Invasion of the cricoid or thyroid cartilage
- Involvement of the tongue base to within 1 cm of the circumvallate papillae.
Vaughan first described the CO2 laser for use in supraglottic squamous cell carcinoma in 1978. Since that time, application of the laser to supraglottic cancer has gained wide acceptance in Europe, but not so in the United States. Some reasons for this may be that the endoscopic approach involves an entirely different treatment paradigm with which most American surgeons are not familiar. In addition, larger lesions are technically more difficult to resect with the laser. And, finally, there has been a proliferation of non-surgical organ preservation protocols in our country.
The key to use of the laser in the supraglottic region is optimizing exposure. A bigger area of exposure is required than for glottal surgery. Steiner started to use a bivalved laryngopharyngoscope in the 1980’s. Zeitels later modified this while maintaining the bivalved design to develop the presently popular supraglottiscope.
Positioning works hand-in-hand with the scope to maximize exposure. The Boyce-Jackson position is optimal: extension occurs at the occipitoatlantic joint with the neck flexed on the chest.
Transoral laser resection is most successful when supraglottic lesions are selected for small size and endoscopic accessibility. The supraglottic lesions most amenable to laser resection are those that rest perpendicular to the distal lumen of the supraglottiscope and therefore minimize tangential cutting. These include
- Suprahyoid epiglottic lesions
- Lesions of the aryepiglottic fold
- Lesions of the false vocal fold
Lesions of the infrahyoid epiglottis and upper FVC are more difficult to resect.
Following resection of the specimen, margin analysis is best accomplished by sending the entire specimen for evaluation. Decisions regarding radiation therapy and management of the necks should be based on the pathology of the primary lesion. Fears that this may result in undesirable treatment delay of the necks should be alleviated by the fact that laser resection can be performed as an excisional biopsy at the time of staging endoscopy.
In Zeitels series of 19 patients with T1 and T2N0 supraglottic lesions limited to sites 1-3 above, none of them failed in the neck, no patient required artificial airway intervention, and most patients returned to a normal diet within several days.
Larger lesions in N0 patients are better served post-excision by full-course XRT to the primary and bilateral necks, and this represents a more aggressive form of treatment than XRT alone, particularly in those patients who may not be good candidates for open surgery. Even in these cases, clear margins are usually obtained at the time of laser excision because of the tendency of supraglottic carcinoma to develop a pseudocapsule. In Zeitels series of 23 patients with T2 or T3N0 lesions treated with laser excision and XRT, 16/23 had clear margins at the primary site; none of these patients failed locally.
However, of the 7 patients without clear margins, 4 experienced local failure requiring salvage total laryngectomy, and another failed in the neck. In general, completely excising the primary lesion prior to XRT is thought to result in a 20-35% treatment advantage over XRT alone. Though Steiner has used single-modality endoscopic treatment for T2 and T3 lesions, most surgeons advocate post-operative XRT because it is extremely difficult to guarantee comprehensive excision of the preepiglottic and paraglottic space.
Ambrosch stated that comparing the results of different treatments for early laryngeal carcinoma one may conclude that laser microsurgery is the method of choice for treatment of these tumors based on oncologic, functional, and economic considerations.
The published results, however, indicate that approximately 70–80% of patients with pT2b and pT3 glottic carcinomas remain free of local tumor recurrence, with minimal morbidity and a functioning larynx. The results of laser microsurgery in patients with moderately advanced supraglottic cancer are comparable to those of open supraglottic laryngectomy with regard to local control and survival rates. They are better than the results published for primary radiotherapy with regard to local control and survival, and they are superior with respect to organ preservation.
Jones et al investigated were 488 patients with T1-2, N0 squamous cell carcinoma of the larynx. Four hundred nineteen patients were treated by irradiation, and 69 were treated with surgery. Most surgical patients were treated earlier in the series, whereas radiotherapy later became the treatment of choice. The primary outcome measures were recurrence at the primary site, recurrence in the neck, and tumor-specific survival. The secondary outcome measure was speech and voice quality. Surgery included horizontal or vertical partial laryngectomy and various minor procedures on the glottis, including cordectomy. Over a 30-year period, radiotherapy was administered to a dose of 60-66 Gy given over 30-33 daily fractions.
They found that surgery tended to be performed early on in the series and radiotherapy thereafter. Surgery was more likely to be carried out for supraglottic disease. These differences apart, the radiotherapy and surgery groups of patients were well matched. The 5-year tumor-specific survival for those treated by irradiation was 87% and for surgery it was 77% (p = .1022). Glottic cancer and T1 disease were associated with high 5-year survivals: 90% and 91%, respectively. Supraglottic site and T2 disease both had a poorer prognoses: 79% and 69%, respectively. The differences for both sets of data were significant. There was no significant difference in primary site recurrence rates for the two treatment modalities, but regional recurrence was higher in the surgery group. Further analysis demonstrated that this was not a function of surgery per se but rather of the unit’s policy toward the N0 neck at the time surgery was carried out. Regarding speech and voice quality, radiotherapy was far superior to surgery. All patients in the radiotherapy group but only 3 of 10 in the surgery group were judged to have a good or normal voice (p = .0017). They concluded that both surgery and irradiation are equally effective at treating early laryngeal carcinoma. Speech and voice were highly significantly better in patients treated by irradiation than in those treated by surgery.
Conclusions
Microendoscopic laser surgery provides an excellent alternative to radiotherapy in the treatment of early-stage glottic cancer. Since its introduction by Strong and Jako in 1972, CO2 laser has found wide acceptance in the treatment of laryngeal diseases. The advantages of laser resection include minimal bleeding, precise control of resection, and the absence of postoperative edema. Cure rates of patients with early-stage glottic carcinoma treated with CO2 laser are equal to those achieved with radiation therapy. Nevertheless, the role and the indications of this technique in the treatment of early-stage glottic cancer has not been defined accurately and remains controversial.
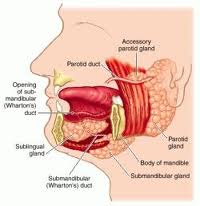
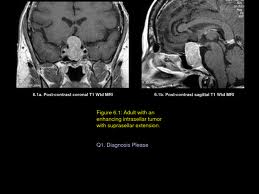
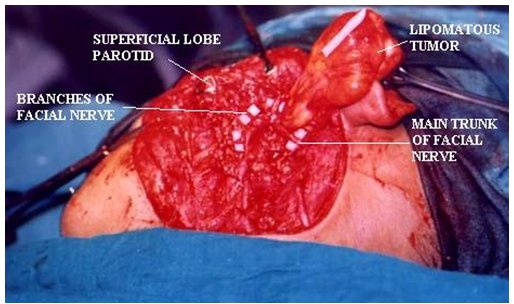
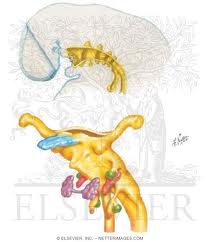
Parapharyngeal Space Tumors
Anatomy
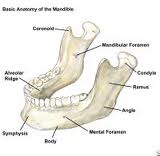
Understanding the anatomy of the parapharyngeal space is important in making a correct diagnosis and surgical plan for excision of tumors in this region. The parapharyngeal space is a potential space, which is often described as being in the shape of an inverted pyramid with the floor at the skull base and it’s tip at the greater cornu of the hyoid bone.
Boundaries
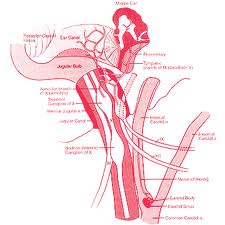
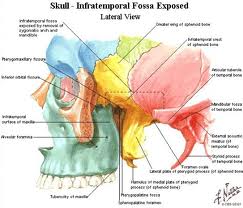
The superior limit of the parapharyngeal space is a small portion of the temporal and bone and the sphenoid bone. It includes the carotid canal, jugular foramen, and hypoglossal foramen. There is a fascial connection from the medial pterygoid plate to the spine of the sphenoid at the superior medial wall, which crosses medial to the foramen ovale and foramen spinosum, which are not included in this space but rather in the infratemporal fossa. The inferior border is the junction of the posterior belly of the digastric muscle and the greater cornu of the hyoid bone. Medially, the boundary is made up of the buccopharyngeal or visceral fascia overlying the superior pharyngeal constrictors. The lateral boundary is made up of the fascia over the medial pterygoid muscle, the ramus of the mandible, the posterior belly of the digastric muscle, and the fascia over the retromandibular deep portion of the parotid gland. Anteriorly the limit is the pterygomandibular raphe. The posterior limit is the dorsal layer of fascia making up the carotid sheath. The internal carotid artery, jugular vein, cranial nerves IX-XII, and sympathetic chain all course through this space.
Prestyloid versus Retrostyloid Tumors
A key anatomical division of the parapharyngeal space is into prestyloid and retrostyloid compartments. Fascia which extends from the styloid process to the tensor veli palantini muscle, called the tensor-vascular-styloid fascia, because it also contains the ascending palatine artery and vein, divides the parapharyngeal space into an anterolateral or prestyloid, and a posteromedial, or retrostyloid compartments. The prestyloid compartment contains fat, a portion of the retromandibular parotid gland, and some lymph nodes. The retrostyloid compartment contains the internal carotid artery, internal jugular vein, cranial nerves IX-XII, sympathetic chain, and lymph nodes. The parapharyngeal lymph nodes have numerous lymphatics, which along with the retropharyngeal nodes drain the soft palate, paranasal sinuses, posterior oral cavity, base of tongue, and a portion of the thyroid gland. The parapharyngeal nodes superiorly are connected to the node of Rouviere in the lateral most retropharyngeal space, this can be a site of metastasis from the nasopharynx, upper oropharynx, and sinuses.
Stylomandibular tunnel
The stylomandibular tunnel is bounded by the posterior ramus of the mandible, the skull base and the stylomandibular ligament. Deep parotid tumors can enter the parapharyngeal space posterior to the stylomandibular ligament resulting in a round shaped lesion or can enter through the stylomandibular tunnel resulting in a “dumbbell” shaped mass.
Pathology
Neoplasms of the parapharyngeal space fall into three categories: primary, metastatic, and extension from adjacent structures. Approximately 80% are benign and 20% malignant. Direct extension can occur from the mandible, maxilla, nasopharynx, neck, oral cavity, oropharynx, and temporal bone. Metastatic tumors include: follicular thyroid carcinoma, medullary thyroid carcinoma, papillary thyroid carcinoma, osteogenic sarcoma, and squamous cell carcinoma. Primary parapharyngeal space neoplasms fall into three categories: salivary gland neoplasms, neurogenic tumors, and miscellaneous tumors.
Salivary Gland Neoplasms
Salivary gland neoplasms are the most common parapharyngeal tumors accounting for 40-50% neoplasms. They are located in the prestyloid space and can arise from either the deep lobe of the parotid gland or from minor salivary glands in the parapharyngeal space. Pleomorphic adenoma is the most common tumor type and mucoepidermoid is the most common malignant neoplasm. Less than 5% of parotid tumors involve the parapharyngeal space. A fat plane on CT or MRI between the mass and the parotid can distinguish a tumor of minor salivary origin from a deep lobe of the parotid tumor. Also, on CT or MRI these lesions displace the carotid artery posteriorly. Masses arising from the deep lobe of the parotid can pass behind the stylomandibular ligament, forming a round tumor, or through the stylomandibular tunnel, forming a “dumbbell” shaped tumor as mentioned previously.
Neurogenic Tumors
The neurilemoma or schwannoma is the most common neurogenic neoplasm of the parapharyngeal space and are the most common retrostyloid masses. The most common site of origin is the vagus nerve, while the sympathetic chain is second. These can also originate from cranial nerves IX, XI, and XII. These lesions are benign and slow growing, often presenting as asymptomatic masses. They generally do not affect their nerve of origin but can extend up through the jugular foramen intracranially. Less than 1% undergo malignant change. By CT or MRI these tumors displace the carotid artery anteriorly.
Paragangliomas (also chemodectomas or glomus tumors) are the second most common neoplasm of the parapharyngeal space. They arise from the nodose ganglion of the vagus nerve, extend into the parapharyngeal space from the carotid body, or extend inferiorly from the jugular bulb. These are also retrostyloid masses. The glomus vagale from the vagus nerve is the most common type. Paragangliomas can be bilateral 10% of the time, and in a patient with a family history of paragangliomas 26-35% of the time. Malignancy has been reported as 10% . These tumors are of neural crest origin and can secrete catecholamines 2% of the time, causing hypertension and flushing. Glomus vagale tumors displace the carotid anteriorly while carotid body tumors will splay the internal and external carotids resulting in the “lyre” sign.
Neurofibromas are the third most common neurogenic tumor of the parapharyngeal space. They originate from the Schwann cells and perineural fibroblasts. These tumors are unencapsulated and intimate involvement of the nerve of origin is common. Sites of origin include the vagus nerve, glossopharyngeal nerve, sympathetic chain, and spinal accessory nerve. This lesions are often multiple and can be associated with Neurofibromatosis type I, in which case there is a higher instance of malignancy.
Miscellaneous Tumors
A wide variety of benign and malignant neoplasms can occur in the parapharyngeal space and account for 20% of lesions. A comprehensive list is beyond this review. Some possible masses include: lymphoma, hemangioma, teratoma, lipoma, branchial cleft cyst, arteriovenous malformation, and internal carotid artery aneurysm.
Clinical Evaluation
The signs and symptoms of parapharyngeal space neoplasms can be subtle and clinical evaluation of this space is difficult. Lesions most often present as an oropharyngeal bulge or as a neck mass. Tumors must reach a size of 2.5-3 cm before becoming palpable as a mass because of the deep location in the neck, so they can reach a large size prior to patient presentation. Other signs and symptoms include: dysphagia, dyspnea, unilateral middle ear effusion, pulsatile tinnitus, bruit, thrill, otalgia, airway obstruction, hoarseness, foreign body sensation, true vocal cord palsy, Horner’s syndrome, dysarthria, and symptoms of catecholamine excess like hypertension and flushing.
All patients should undergo a comprehensive history and complete head and neck examination. If the lesion is large enough to be palpated, bimanual palpation is important. Paragangliomas are classically described as compressible and mobile in an anterior-posterior direction but not in a vertical direction. Patients in whom a paraganglioma is suspected should undergo a 24-hour urine collection for vanillymandelic acid and metanephrines. If urinary catecholamines are elevated a metaiodinated benzylguanidine (MIBG) scan should be obtained to look for multiple lesions. In some cases fine needle aspiration of the lesion can be useful if a malignant tumor is suspected, however this should only be performed after imaging to rule out a vascular lesion.
Imaging
CT, MRI, and angiography are all used to evaluate parapharyngeal neoplasms. A CT or MRI is most commonly the first study obtained. Angiography is performed for all enhancing lesions.
CT Scan
CT scan with and without contrast is useful in the evaluation of parapharyngeal masses. Information obtained from this study is the location of a tumor in either the prestyloid or retrostyloid compartments, the presence or absence of a fat plane between the deep lobe of the parotid and a prestyloid mass, and if the mass enhances with contrast. Schwannomas enhance because of the extravascular accumulation of contrast while a paraganglioma is a hypervascular mass. Displacement of the internal carotid artery posteriorly is characteristic of prestyloid tumors while displacement anteriorly is indicative of a poststyloid mass. Bone erosion by malignant tumors can be seen. CT does have limitations in soft tissue detail in comparison to MRI and exposes the patient to radiation. If a retrostyloid mass is found or if malignancy is suspected an MRI should be obtained.
MRI
 Soft tissue resolution is better with MRI than with CT scan. It is probably the most useful study to evaluate parapharyngeal neoplasms. The relationship between the mass and the carotid can be more clearly seen with MRI than with CT scan. There are characteristic appearances of different tumors on MRI scan. Pleomorphic adenomas have low signal intensity on T1 images and high on T2 images and displace the carotid posteriorly. Schwannomas have higher signal intensity on T2 images like pleomorphic adenomas, but displace the carotid anteriorly. Paraganliomas have a characteristic “salt and pepper” appearance on T2 weighted images because of flow voids.
Soft tissue resolution is better with MRI than with CT scan. It is probably the most useful study to evaluate parapharyngeal neoplasms. The relationship between the mass and the carotid can be more clearly seen with MRI than with CT scan. There are characteristic appearances of different tumors on MRI scan. Pleomorphic adenomas have low signal intensity on T1 images and high on T2 images and displace the carotid posteriorly. Schwannomas have higher signal intensity on T2 images like pleomorphic adenomas, but displace the carotid anteriorly. Paraganliomas have a characteristic “salt and pepper” appearance on T2 weighted images because of flow voids.
Angiography
In the past all enhancing lesions were evaluated with angiography. Advances in MRI imaging have decreased the use of angiography in these tumors. This study can demonstrate the relationship of the tumor to the great vessels and distinguish between neurogenic and vascular lesions. Carotid body tumors cause splaying at the bifurcation resulting in the “lyre” sign mentioned above. Carotid artery balloon occlusion tests should be performed if malignancy is suspected and there is the possibility of carotid sacrifice. Tumor embolization can be performed for paragangliomas 1 day prior to surgery to reduce blood supply. Some authors report that this increases fibrosis around the tumor making dissection more difficult, and do not use it.
Surgical Approaches
Transoral
The transoral approach has been used for selected small benign lesions of the prestyloid space presenting as an oropharyngeal mass. Problems with this approach are limited exposure, increased risk of tumor spillage, and possibility of neurovascular injury.
Cervical with or without mandibulotomy
Approach recommended by many authors. A transverse incision at the level of the hyoid bone with either removal or displacement of the submandibular gland is performed. Incision of the fascia deep to the posterior submandibular gland is incised. To increase exposure the digastric muscle, stylohyoid, and styloglossus muscles can be released from the hyoid bone and the stylomandibular ligament can be divided. Tumors may often be bluntly dissected with a finger.
Further exposure can be obtained by performing mandibulotomy of the body, ramus or the angle. Tracheostomy is necessary with this approach. After removal of the tumor the mandibulotomy is repaired with plates and screws.
Cervical-Parotid
A cervical approach incision may be extended superiorly in front of the ear. This allows identification of the facial nerve trunk and lower division of the facial nerve. The posterior belly of the digastric muscle is divided exposing the internal carotid, jugular vein, and nerves. The stylomandibular ligament, styloglossus muscle, and stylohyoid muscle are divided close to the styloid process. This approach may be combined with mandibulotomy to improve exposure.
Transparotid
For deep lobe parotid tumors a transparotid approach may be used. A superficial parotidectomy is performed and the facial nerve retracted. Dissection is carried around the mandible. If necessary, mandibulotomy may be used posterior to the entrance of the inferior alveolar nerve.
Cervical-Transpharyngeal
 For large or highly vascular tumors a cervical incision is extended to midline lip incision. Mandibulotomy is performed either in the midline or anterior to the mental foramen and the incision is carried along the floor up to the anterior tonsillar pillar. The mandible is swung laterally and the tonsil and superior constrictors moved medially. This gives wide access to the parapharyngeal space. Tracheostomy is necessary.
For large or highly vascular tumors a cervical incision is extended to midline lip incision. Mandibulotomy is performed either in the midline or anterior to the mental foramen and the incision is carried along the floor up to the anterior tonsillar pillar. The mandible is swung laterally and the tonsil and superior constrictors moved medially. This gives wide access to the parapharyngeal space. Tracheostomy is necessary.
Infratemporal Fossa
A preauricular lateral infratemporal fossa approach may be used for tumors involving the skull base or extending into the infratemporal fossa. This may be combined with a frontotemporal craniotomy for tumors with significant intracranial extension.
Transcervical-Transmastoid
The cervical incision is extended postauricularly and a mastoidectomy is performed. The mastoid tip is removed and the jugular fossa exposed. Sometimes the facial nerve may need to be dissected out of the fallopian canal and transposed. Transient facial paresis should be expected.
Nonsurgical Management
In some cases of patients who are poor surgical candidates, who fail balloon occlusion, are elderly, have unresectable lesions, or have benign slow growing tumors that would require sacrifice of multiple cranial nerves surgery may not be the only option. Other options include observation, or for paragangliomas, radiation. Paragangliomas tend to grow 1.0-1.5 mm per year. Mortality is less than 10% per year for untreated paragangliomas. Although radiation is not curable for paragangliomas, local control rates of 96-100% have been reported.
Conclusions
The parapharyngeal space is an area of complex anatomy where clinical exam is difficult. The surgeon must understand the pathology of tumors of this space and the proper use of imaging studies to make a preoperative diagnosis. This allows for planning of a surgical approach and preoperative counseling.
Laryngeal Trauma
Anatomy and Physiology
Fortunately the larynx is well protected by the mandible, the sternum and the flexion mechanism of the neck. The primary functions of the larynx are to provide an airway, protect the lower respiratory tract, and produce the voice. The larynx can be divided into three areas: supraglottis, glottis and subglottis. Support is maintained by the hyoid bone, thyroid cartilage and cricoid cartilage. The supraglottis is less dependent on external support and contains abundant soft tissue and redundant mucosa. The glottis relies heavily on external support and the coordination of cricoarytenoid mobility and neuromuscular activity to support the airway and provide phonation. In the adult, the airway is narrowest at the glottis. Therefore, injury at this level may seriously compromise airway support. The subglottis is supported by the only circular cartilage in the larynx, the cricoid, which is the narrowest point of the neonatal and infant airways.
Mechanism of Injury
The type of injury can be classified as either blunt or penetrating. Blunt injuries are commonly the result of motor vehicle crashes. The laryngeal skeleton is compressed between a foreign object (i.e., steering wheel or dashboard) and the anterior aspect of the cervical spine. This splays the alae of the thyroid cartilage and with enough force, may fracture the cartilage often in a vertical median or paramedian nature. Other types of blunt trauma include sports related injury, strangulation, assaults or the clothesline injury associated with snowmobiles or all terrain vehicles. The force of the injury can cause significant soft tissue and/or cartilage disruption with minimal external signs of trauma. Blunt trauma may also cause dislocation of the cricoarytenoid joint or damage to the recurrent laryngeal nerves with resultant impaired vocal cord mobility.
Penetrating trauma to the larynx may occur secondary to knife or bullet wounds. The extent of injury varies with the type of assault weapon, and it is important to realize that other associated injuries, such as neurological, vascular, or esophageal, are more common in these situations.
Initial Evaluation
Initial management should follow ATLS principles. Securing an airway takes precedence over other problems. Injury to the cervical spine should be suspected in these patients until proven otherwise. There is some controversy over airway management in these patients, but most authors recommend tracheotomy under local anesthesia for patients exhibiting respiratory distress. Attempts at oral or nasotracheal intubations in these patients may result in further damage to an already tenuous airway. Cricothyroidotomies should be avoided in the setting of laryngeal trauma as this may contribute to further injury. Special considerations exist in the pediatric patient population. Due to the smaller dimensions of the pediatric airway and potential for soft swelling edema with laryngeal trauma, it is recommended that they undergo tracheotomy over a ventilating bronchoscope in the operating room. In patients with no acute breathing difficulties, a detailed history and careful physical examination can be obtained.
Presenting symptoms of laryngeal trauma may include a change in the quality of voice, pain, dysphagia, odynophagia, hemoptysis and/or stridor. Inability to tolerate the supine position is a concerning symptom with regard to airway stability. Schaefer reports that the symptom correlating with the most severe injury is impaired respiration. Fuhrman, et al., reports the most reliable symptom as hoarseness. Classic signs of external laryngeal trauma include hoarseness, subcutaneous emphysema and hemoptysis. The most reliable symptoms reported by Fuhrman were tenderness and subcutaneous emphysema. Other physical exam findings, such as anterior neck contusion and tracheal deviation may also occur. Associated injuries including cervical spine, esophageal and vascular injuries must be considered and evaluated.
In stable patients, flexible fiberoptic laryngoscopy in the emergency room should be performed. CT scan, direct laryngoscopy, bronchoscopy and esophagosopy are used selectively based on initial fiberoptic exam findings. It has become increasingly important to categorize patients by the severity of trauma according to fiberoptic exam and CT findings in order to aid with the management of their injuries. Suggested protocols for treatment are offered by categorization into four groups established by Schaefer and Close with the addition of a fifth group for cricotracheal separation by Fuhrman et al.
Laryngotracheal Injury Classification
Group I injuries include minor endolaryngeal hematoma, edema or laceration without detectable fracture. Group II injuries have edema, hematoma, minor mucosal disruption without exposed cartilage, and nondisplaced fractures noted on CT scan. Massive edema, mucosal disruption, displaced fractures, exposed cartilage and/or cord immobility qualify as Group III injuries. Group IV injury is the same as group III with the addition of two or more fracture lines, skeletal instability or significant anterior commissure trauma. The group V category includes complete laryngotracheal separation.
Radiologic evaluation
In the stable patient, after the airway has been evaluated, a CT scan of the larynx can be obtained. A CT scan is not necessary in a patient presenting with clear indications for surgery, such as active bleeding, hemoptysis, need for emergent surgical airway, exposed cartilage and significant lacerations on laryngoscopy, or air escaping through the neck wound. Conversely, patients with minimal trauma and a normal exam will not likely benefit from a CT scan of the larynx. In patients with significant laryngeal trauma and intermediate exam findings, CT scanning should be performed to determine the integrity of the laryngeal framework. It should be noted that some authors routinely obtain CT scans even for severe trauma before proceeding to the operating room as a “roadmap” of the patient’s injuries. The entire cervical spine should be evaluated radiographically. Angiography and Gastrografin esophagrams may be indicated in selected cases, especially in the event of significant penetrating trauma to the area.
Nonsurgical Management
Laryngeal injuries may be treated medically or surgically depending on initial fiberoptic laryngoscopic and CT scan findings. A patient can be treated medically with close observation if the injury will resolve without surgical intervention and the airway is stable. Group I injuries can be safely managed with a minimum of 24 hours of close observation, head of bed elevation, voice rest and humidification of inspired air. Antibiotics are recommended with disruption of the laryngeal mucosa. Treatment with anti-reflux medication is also initiated. Although not proven, systemic steroids are often given to reduce laryngeal edema. Nasogastric tube feedings should be considered if significant mucosal lacerations are present. Serial flexible fiberoptic examinations should be performed to evaluate the airway and healing prior to discharge.
Surgical Management
The indications for surgical management may range from the need to establish an airway to open reduction and internal fixation of laryngoskeletal fractures. Penetrating traumas are more likely to require open exploration than blunt traumas. Group II through group V patients will usually require some form of surgical intervention. Surgical options fall into one of three categories: endoscopy alone, endoscopy with exploration, and endoscopy with exploration and stenting. If there is any doubt about the extent of injury endoscopy should be performed. Indications for surgical exploration include: large mucosal lacerations, exposed cartilage, multiple or displaced cartilaginous fractures, vocal cord immobility, fractured cricoid, disruption of the cricoarytenoid joint, and lacerations involving the free margin of the vocal cord or anterior commisure. A vertically oriented fracture of the median or paramedian thyroid ala may significantly alter the stability of the laryngeal skeleton and usually necessitates ORIF. Tracheotomy should be performed in patients with injuries of this extent and should be lower
than usual (fourth to fifth ring) via a vertical incision (better exposure in the case of laryngotracheal separation).When laryngeal exploration is indicated, it should be performed within 24 hours of the injury in order to maximize airway and phonation results. A horizontal skin incision is made at the level of the cricothyroid membrane and subplatysmal flaps are elevated. The strap muscles are then divided in the midline and the laryngeal skeleton is exposed. The larynx can then be explored via a midline thyrotomy or via a vertical fracture within 2 to 3 mm of the midline. The thyroid laminae are retracted laterally to visualize the endolarynx. All exposed cartilaginous and submucosal tissues are then covered with mucosa working posteriorly to anteriorly. Primary closure is almost always possible and debridement should be minimized. Closure is performed with absorbable suture with knots outside the laryngeal lumen to prevent granulation. Displaced arytenoid cartilages should be reduced. The anterior commissure should be reconstituted by using 4.0 sutures to suspend the anterior true vocal cords to the outer perichondrium of the thyroid cartilage. The thyrotomy can then be reapproximated with nonabsorbable suture, wire or rigid miniplates.
Fractures of the cartilages are reduced and can be stabilized using a variety of materials, including stainless steel wires, nonabsorbable suture, and miniplates. If the fracture is comminuted, small fragments of cartilage with no intact perichondrium are removed to prevent chondritis. Adaptation miniplates have the theoretical advantage of immediate stability of the larynx (less need for endolaryngeal stenting), ability to bridge large gaps (comminuted fractures), and easier restoration of the preinjury geometry of the laryngeal framework.
Endolaryngeal stenting is reserved for wounds involving disruption of the anterior commissure, massive mucosal injuries and comminuted fractures of the laryngeal skeleton. Stenting reestablishes the normal scaphoid shape of the anterior commissure, stabilizes severely comminuted fractures and prevents web formation and stenosis. A variety of stents can be used including a shortened Portex endotracheal tube, manufactured silastic stents, or a finger cot filled with sponge rubber. Stents should reach from the false cords to the first tracheal ring and conform to the shape of the endolarynx. The stent should be stabilized within the larynx and allow movement with the larynx during swallowing. A heavy, nonabsorbable suture is passed through the stent and larynx at the ventricle and another at the cricothyroid membrane and tied over buttons outside the skin. The stent should not be left longer than 2 to 3 weeks. Removal is performed under general anesthesia with endoscopy and additional procedures for removal of granulation tissue as necessary.
The unique injury of laryngotracheal separation usually results in immediate death. Occasionally the airway may maintain patency with an intact mucosal layer. Although successful intubation attempts with bronchoscopy have been reported, it is usually more safely managed by emergent tracheotomy. Bilateral recurrent laryngeal nerve injury and subglottic stenosis are common with this injury. Surgical repair requires permanent sutures between the cricoid and second tracheal ring for airway support. This may be difficult with concomitant cricoid fracture after internal fixation.
Severe wounds involving extensive tissue and framework loss of the supraglottic or hemilarynx can be managed using various partial laryngectomy procedures to restore function.
Total laryngectomy is a last resort and is reserved for situations where the basic elements of the laryngeal skeleton and investing soft tissue are not usable for repair.
Outcomes
The ultimate quality of airway, voice and swallowing are important considerations following repair of external laryngeal trauma. Airway status is poor if the patient remains cannulated, fair with mild aspiration or exercise intolerance and good if it resembles preinjury status. Voice can be considered poor if it represents aphonia or whisper, fair if it is functional but changed (hoarse), and good if normal. Swallowing function is either normal or abnormal from the subjective reports of the patient. Generally, conservatively managed injuries fare better than surgical ones in large part due to the differences in severity of the initial insult. The use of endolaryngeal stents tends to decrease the quality of voice without affecting the overall airway status. Also, the less time the stent is left in place, the more favorable the result. Vocal cord paralysis adversely affects outcomes with regard to both airway and voice. Improved results are also shown with the earlier the timing of the repair (less than 48 hours from injury being significant).
Conclusion
External laryngeal trauma is a rare injury that can be managed in a systematic fashion. Early recognition is important for both initial preservation of life as well as long-term airway and vocal function. The signs and symptoms hoarseness, subcutaneous emphysema, and pain with a history of laryngeal trauma should prompt a timely evaluation of the larynx and airway support. Flexible fiberoptic laryngoscopy followed by CT scans and surgical procedures as deemed necessary are standard in the initial evaluation. Associated cervical spine, vascular and esophageal injuries should be excluded. Treatment, either medical or surgical (with/without stenting) is based on the site and extent of injury.
Laryngeal Trauma
Laryngeal embryology
The larynx develops from the fourth and fifth branchial arches. At the third week of gestation, the respiratory primordium is derived from the primitive foregut to later form the lung bud and later the bronchial bud which will eventually develop into the tracheobronchial tree. At the fourth and fifth week of gestation the tracheoesophageal folds fuse to form the tracheoesophageal septum leading to the separation of the tracheal airway lumen from the esophageal digestive tract.
The laryngotracheal groove is the primitive opening of the larynx during development. This structure will develop into the primitive laryngeal aditus which is formed by three eminences. The hypobranchial eminence is the most cephalad of these structures and will later develop into the epiglottis. The other two eminences will form the arythenoid cartilages. The laryngeal lumen obliterates and later recanalyzes by the tenth week of gestation.
There are certain difference between the adult and the newborn larynx. The diameter of the subglottic and glottis are narrower which leads to increased propensity for airway obstruction and compromise. In the infant the narrowest portion of the airway is the subglottis in contrast to the glottis in the child and adult. The subglottic region is about 4 to 5 millimeters in diameter. The epiglottis is also narrower in infants and is tubular and omega in shape. The larynx lies at the level of the fourth cervical vertebrae at birth. By fifteen years of age it descends to the level of the six to seventh vertebrae. Blunt laryngotracheal injury is uncommon in the pediatric population because the mandible, the elasticity of the cartilaginous support of the airway, and the mobility of the supporting tissues collectively act to protect the laryngotrachea.
Laryngeal Anatomy
The larynx can be divided into three areas: supraglottis, glottis and subglottis. Support is maintained by the hyoid bone, thyroid cartilage and cricoid cartilage. The supraglottis is less dependent on external support and contains abundant soft tissue and redundant mucosa. The glottis relies heavily on external support and the coordination of cricoarytenoid mobility and neuromuscular activity to support the airway and provide phonation. In the adult, the airway is narrowest at the glottis. Therefore, injury at this level may seriously compromise airway support. The subglottis is supported by the only circular cartilage in the larynx, the cricoid, which is the narrowest point of the neonatal and infant airways.
The anatomy of the larynx and the relation to adjacent structures are key to the rarity of injury. The inferior projection of the mandible affords significant protection from anterior blows. Inferiorly the larynx is protected by the sternum and laterally by the sternomastoid muscle. Posteriorly, the larynx is protected by the cervical spine.
Laryngeal Function
The larynx has an array of functions including breathing passage, airway protection, clearance of secretions, and vocalization. To maintain these functions, the skeletal framework and its underlying soft tissue must be intact. The framework includes the thyroid cartilage, cricoid cartilage and the tracheal rings. In laryngeal injuries, symptoms are commonly direct manifestation of the malfunction of the coordinated activities within the larynx. Reduction in the size of the laryngeal airway can produce symptoms of airway obstruction ranging from mild stridor, to increased work of breathing associated to retractions, nasal flaring and tachypnea, apnea, cyanosis and even sudden death.
Damage to the cricoarytenoid joint or damage to the recurrent laryngeal nerves with resultant impaired vocal cord mobility may lead to aspiration and dysphonia. These symptoms persist after resolution of acute life threatening period affecting the patient’s long-term quality of life.
Mechanism of Injury
The mechanisms of laryngeal injury can be divided into blunt trauma, including clothesline, crushing, and strangulation injuries; penetrating trauma; inhalation injuries; and injuries caused by caustic ingestions.
Anterior blunt injuries are most commonly the result of motor vehicle accidents. This can be a car driver, which on forward thrust during rapid deceleration the neck is extended impacting against the steering wheel. The laryngeal skeleton is compressed between a foreign object (i.e., steering wheel or dashboard) and the anterior aspect of the cervical spine. Hyperextension of the neck removes the mandibular barrier, exposing the larynx to anterior crushing forces. With the increasing use of seatbelts and airbags, the number of laryngeal fractures arising from motor vehicle accidents is likely to decrease, although seat belt harness and air bags injuries of the larynx have been described. Blunt pediatric neck injuries are more often life-threatening, because relatively minor direct trauma to the larynx or trachea may result in significant injury, including laryngotracheal disruption. . Also, the relative smaller cross-sectional area of the pediatric population predisposed them to higher risk of airway compromised.
Other less common blunt injuries can be caused by the motorcyclist or snowmobile who suddenly encounters a fixed horizontal barrier at the level of the neck suffering a clothes-line type injury. This type of injury imparts a large amount of energy over a relatively small area, resulting in massive trauma and a high probability of sudden death by crushing the larynx or separating the cricoid from the larynx or trachea. Assault accounts for a small proportion of cases. Strangulation by assault or during a suicidal attempt may lead to immediate airway obstruction or delayed laryngeal with airway compromised.
Sport-related injuries occur most frequently in high velocity sports such as cycling, motorcycle racing and ice hockey or in the martial arts. The incidence is dependent on the popularity of the particular sport.
Penetrating trauma to the larynx most commonly occur secondary to knife or bullet wounds. In gunshot wounds, the extent of injury could be the result of direct penetration or indirectly by the blast effect which varies with the type of assault weapon.
Initial Evaluation
Initial management should follow ATLS principles. Securing an airway takes precedence over other problems and injury to the cervical spine is assumed until proven otherwise. In laryngeal trauma there is controversy about the method of securing the airway. The American College of Surgeons recommends an attempt at intubation, failing which an emergency tracheostomy should be performed. However, Schaefer has stated that intubation following laryngeal trauma is hazardous. Most authors recommend tracheotomy under local anesthesia for patients exhibiting respiratory distress. Intubation is often impossible to perform in severe trauma, due to swelling and bleeding. It may also worsen a preexisting injury, possibly causing further tears or cricotracheal separation. Even among those who suggest intubation as the initial method of airway control, it is emphasized that it should be done by an experienced physician(Hwang 2004). Cricothyroidotomies should be avoided in the setting of laryngeal trauma as this may contribute to further injury.
Special considerations exist in the pediatric patient population. The option of local tracheotomy is not feasible in a frightened, injured child. The time margin of error is also less because the arterial oxygen saturation drops more quickly than in an adult. In this instance, rigid bronchoscopy is performed to secure the airway under direct visualization. A tracheotomy can then be performed over the bronchoscope.
In patients with no acute breathing difficulties, a detailed history and careful physical examination can be obtained. Observation without tracheotomy or endotracheal intubation may be indicated.
Diagnosis
Presenting symptoms of laryngeal trauma may include a change in the quality of voice, pain, dyspnea, Subcutaneous emphysema, dysphagia, odynophagia, hemoptysis and/or stridor. Inability to tolerate the supine position is a concerning symptom with regard to airway stability. On physical exam the vitals signs including respiratory rate and saturations are closely monitored. The skin of the neck may reveal contusions or abrasions in blunt trauma or a line pattern indicative of a strangulation injury. Some obvious signs of injury are tracheal deviation, open wound with air bubbles or tracheal cartilage. Any penetrating injury is examined for an entrance and exit wound, and the most likely path of travel of the projectile is determined. Open wounds are not explored with instruments, nor are they probed for fear of dislodging a hematoma and initiating further bleeding.
In stable patients, flexible fiberoptic laryngoscopy in the emergency room should be performed. CT scan, direct laryngoscopy, bronchoscopy and esophagosopy are used selectively based on initial fiberoptic exam findings. It has become increasingly important to categorize patients by the severity of trauma according to fiberoptic exam and CT findings in order to aid with the management of their injuries. CT allows evaluation of the laryngeal skeletal framework in a noninvasive manner, thus avoiding unnecessary operative explorations by selecting patients who should do well without surgical intervention. Optimal imaging is performed using spiral technique and subsecond scan times, particularly when using two-dimensional sections for multiple projections or three-dimensional reconstructions. CT should be reserved for patients in whom laryngeal injury is suspected by history and physical examination without obvious surgical indications.
Laryngotracheal Injury Classification
Group I injuries include minor endolaryngeal hematoma, edema or laceration without detectable fracture. Group II injuries have edema, hematoma, minor mucosal disruption without exposed cartilage, and nondisplaced fractures noted on CT scan. Massive edema, mucosal disruption, displaced fractures, exposed cartilage and/or cord immobility qualify as Group III injuries. Group IV injury is the same as group III with the addition of two or more fracture lines, skeletal instability or significant anterior commissure trauma. The group V category includes complete laryngotracheal separation.
Medical Management
The purpose of the extensive physical and radiologic evaluation is to identify the patients with injury and to select patients who are likely to do well without surgical intervention. A patient can be treated medically with close observation if the injury will resolve without surgical intervention and the airway is stable. Group I injuries can be safely managed with a minimum of 24 hours of close observation, head of bed elevation, voice rest and humidification of inspired air. Antibiotics are recommended with disruption of the laryngeal mucosa. Treatment with anti-reflux medication is also initiated. Nasogastric tube feedings should be considered if significant mucosal lacerations are present. Serial flexible fiberoptic examinations should be performed to evaluate the airway and healing prior to discharge. Although not proven, early systemic steroids therapy are often given to reduce laryngeal edema. There was one randomized controlled trial on the use of intravenous dexamethasone in preventing traumatic laryngeal edema in pediatric bronchoscopy. This study showed no reduction in postbronchoscopy laryngeal oedema with the use of intravenous dexamethasone.
Surgical Management
The principles of surgery are hemostasis, evacuation of hematoma, reconstruction of the laryngeal framework and coverage of de-epithelialized surfaces. Group II through group V patients will usually require some form of surgical intervention. Surgical options fall into one of three categories: endoscopy alone, endoscopy with exploration, and endoscopy with exploration and stenting. If there is any doubt about the extent of injury endoscopy should be performed. Indications for surgical exploration include: large mucosal lacerations, exposed cartilage, multiple or displaced cartilaginous fractures, vocal cord immobility, fractured cricoid, disruption of the cricoarytenoid joint, and lacerations involving the free margin of the vocal cord or anterior commisure.
When laryngeal exploration is indicated, it should be performed within 24 hours of the injury in order to maximize airway and phonation results. A horizontal skin incision is made at the level of the cricothyroid membrane and subplatysmal flaps are elevated. The strap muscles are then divided in the midline and the laryngeal skeleton is exposed to the level of the hyoid superiorly and sternal notch inferiorly. The larynx can then be explored via a midline thyrotomy or via a vertical fracture within 2 to 3 mm of the midline. Simple nondisplaced fractures can be repaired by suturing the outer perichondrium with nonabsorbable sutures. Primary closure is almost always possible and debridement should be minimized. All mucosal lacerations are meticulously repaired using fine absorbable sutures. Closure is performed with knots outside the laryngeal lumen to prevent granulation. Displaced arytenoid cartilages should be reduced. The anterior commissure should be reconstituted by using 4.0 sutures to suspend the anterior true vocal cords to the outer perichondrium of the thyroid cartilage. Fractures of the cartilages are reduced and can be stabilized using a variety of materials, including stainless steel wires, nonabsorbable suture, and miniplates. If the fracture is comminuted, small fragments of cartilage with no intact perichondrium are removed to prevent chondritis. The thyrotomy can then be reapproximated with nonabsorbable suture, wire or rigid miniplates.
There may be a need for endolaryngeal stenting. Stents that have reportedly been used include cut portions of an endotracheal tube as well as finger cots filled with gauze or foam to commercially manufactured polymeric silicone stents. Endolaryngeal stenting is reserved for wounds involving disruption of the anterior commissure, massive mucosal injuries and comminuted fractures of the laryngeal skeleton. Stenting reestablishes the normal scaphoid shape of the anterior commissure, stabilizes severely comminuted fractures and prevents web formation and stenosis. The stent should extend from the false vocal fold to the first tracheal ring to add stability and prevent endolaryngeal adhesions. The stent should be secured in such a manner as to be easily removed using endoscopic techniques. The stents should be removed in a period of 10 to 14 days to prevent mucosal damage, even in the case of severe injury.
In the case of laryngotracheal separation, surgical repair requires permanent sutures between the cricoid and second tracheal ring for airway support. Bilateral recurrent laryngeal nerve injury and subglottic stenosis are common with this injury. If the recurrent laryngeal nerve is severed by the injury, a neurorrhaphy of the severed ends should be performed.
Severe wounds involving extensive tissue and framework loss of the supraglottic or hemilarynx can be managed using various partial laryngectomy procedures to restore function. Total laryngectomy is a last resort and is reserved for situations where the basic elements of the laryngeal skeleton and investing soft tissue are not usable for repair.
Outcomes
The outcome after laryngeal trauma depends on the extent of the original injury and the quality of subsequent repairs. The ultimate quality of airway, voice and swallowing are important considerations following repair of external laryngeal trauma. Airway status is poor if the patient remains cannulated, fair with mild aspiration or exercise intolerance and good if it resembles preinjury status. Voice can be considered poor if it represents aphonia or whisper, fair if it is functional but changed (hoarse), and good if normal. Swallowing function is either normal or abnormal from the subjective reports of the patient. Vocal cord paralysis adversely affects outcomes with regard to both airway and voice. Improved results are also shown with the earlier the timing of the repair (less than 48 hours from injury being significant).
Conclusion
Although laryngeal trauma is an uncommon injury, it is life-threatening. Recognizing any airway compromise and need for immediate intervention could prevent immediate death as well as acute and long term morbidity. Initial management should follow ATLS principles. In laryngeal trauma there is controversy about the most appropriate method of securing the airway in certain scenarios, but most authors agree that tracheotomy should be performed on patients exhibiting respiratory distress. In patients with no acute breathing difficulties, a detailed history, careful physical examination and appropriate diagnostic tools should be use to differentiate the need for medical from surgical management.
Pediatric Airway Emergencies
Anatomy
The complex structures of the human airway vary in anatomy and physiology from birth to adulthood. The infant’s larynx and trachea are significantly smaller than the adult’s. The vocal cords of the newborn infant are 6-8 mm long and the vocal processes of the arytenoids extend one half of that length. The posterior glottis’ transverse length is approximately 4 mm. The subglottis has a diameter of between 5 and 7 mm. The trachea itself is about 4 cm long and as a diameter of 3.6 mm. These dimensions leave little margin for obstruction in the infant, unlike the adult. The area of a circle is equal to the square of the radius, therefore a very small change in mucosal edema can lead to significant changes in airway caliber.
The infant’s oral airway differs from the adult’s as well. The infant’s epiglottis nearly touches the soft palate. Also, the infant’s tongue is larger than that of the adult, and generally occupies all of the space in the infant’s mouth. These factors contribute to making the infant a preferential nasal breather for 2 to 5 months. Because infants are nasal breathers, nasal obstruction can lead to significant respiratory distress. The act of oral breathing is a reflex that often does not develop at all until several weeks after birth. The nasopharyngeal airway of the infant and child is significantly narrower than that of the adult because of the increased relative size of the child’s adenoid pad, lingual and palatine tonsils. The coordination of swallowing is usually well developed in a full term infant. However, before the 34th week of gestation, the pre-term infant generally demonstrates a poorly coordinated suckling response and may be unable to protect the airway.
History
The first step in evaluation of the child with airway difficulty is assessment of the urgency of the situation. Immediate history and physical examination should proceed simultaneously until the general status of the patient is known. Once it is established that the child is not in immediately life-threatening respiratory distress, the examiner should take a thorough medical history. It is very important to ask about the onset of respiratory distress. For instance, sudden onset of choking followed by respiratory distress is strongly indicative of foreign body aspiration. The examiner should always specifically ask about a history of foreign body ingestion or aspiration. Knowledge of the age at onset of symptoms can narrow the diagnostic possibilities, as discussed below. The family should be questioned about noisy breathing, the types and associations of which are discussed below. The family should be asked about aggravating factors, such as feeding or sleeping. Respiratory distress associated with feeding may indicate the presence of aspiration or of nasal airway compromise. The examiner should note previous history of intubation and surgical interventions. Respiratory distress or stridor immediately after extubation may be due to subglottic edema whereas distress that starts after two to three weeks may indicate early subglottic stenosis or vocal cord granuloma. The investigator should ask about throat or neck pain and fever, indicating inflammatory causes of airway obstruction. Birth history should be carefully explored, including history of birth trauma, as vocal cord paralysis due to birth trauma is a common cause of respiratory distress or stridor in infants. History of other congenital anomalies should also be noted.
Physical Examination
The physical examination should begin with careful inspection of the patient. The patient can remain in the caregiver’s arms while the examiner assesses the respiratory rate and the degree of distress. Increased rate of respiration, nasal flaring, use of accessory muscles or presence of respiratory fatigue can indicate serious respiratory distress. Cyanosis is usually a very late sign of respiratory compromise. The above findings, especially if progressive, often indicate a need for immediate airway stabilization (for example, by endoscopy followed by intubation).
In a stable child, the examination can proceed. Auscultation of the noises made by the child on respiration is helpful in determining the level of the obstruction. Patients with nasal obstruction generally have a normal voice or cry, except when the obstruction is severe, when the voice becomes hyponasal. Nasal flaring and snorting is a frequent finding, with chest retractions noted in severe nasal compromise. Neonates, who are obligate nasal breathers, often experience serious respiratory distress with nasal obstruction. Older children with nasal obstruction characteristically breathe with their mouths open. Most children with nasal obstruction will experience some respiratory distress during feeding. Auscultation of each nare with the cord of a stethoscope is often useful to determine whether there is any airflow through either side individually.
Patients with oropharyngeal obstruction usually have a normal voice, though it may be throaty or full. They usually present with inspiratory stridor that is coarse (also known as stertor) and increases during sleep. Sternal and intercostal retractions are common and may increase to total chest retractions. Feeding is often difficult or impossible, and patients may be unable to handle their own saliva. These patients, like those with nasal obstruction, tend to breathe with their mouths open and jaw forward. As with nasal obstruction, cough is usually not a symptom.
Patients with supraglottic laryngeal obstruction often present with a muffled or throaty voice. These patients tend to snore while sleeping and produce coarse inspiratory sounds at rest. Feeding is difficult for these patients and mouth breathing is the norm. Cough is not usually present. Patients with glottic obstruction are usually hoarse and may even be aphonic. They usually have an inspiratory stridor initially, but will develop biphasic stridor with progression. Chest retractions are common with severe obstruction, but mouth breathing and coughing are not. Patients with subglottic obstruction may have a hoarse, husky, or even normal voice. These patients generally have an inspiratory stridor that can progress to biphasic. Feeding is normal and nose breathing is the norm. These patients often have a barking cough. Patients with tracheobronchial obstruction usually have a normal voice and their stridor is generally expiratory with a component of wheezing. Feeding is uncommon, and a brassy cough is usually present.
After observation and auscultation of the patient with respiratory difficulty, the examiner should attempt to reposition the patient to determine the effect on noisy breathing. Noisy breathing caused by laryngomalacia, micrognathia, macrogossia, and innominate artery compression diminishes when the baby lies prone with the neck extended. Respiratory distress caused by unilateral vocal cord paralysis may improve with the baby lying on the affected side.
Flexible laryngoscopy is usually, but not always, required for the complete evaluation of the infant or child with respiratory obstruction. Only uncommonly is indirect laryngoscopy with a mirror successful. For flexible laryngoscopy, the examiner should have suction, oxygen, and bag ventilation kits available, as flexible laryngoscopy in small children may cause apnea by unclear pathways. The exam should begin with bilateral evaluation of the nasal cavities, choanae and nasopharynx. The vocal cords should be visualized and their mobility documented, and pooling of secretions should be noted in the hypopharynx. Findings of laryngopharyngeal reflux such as posterior glottic edema, hypervascularity, and pseudocsulcus should be noted. The flexible laryngoscope is generally not useful for detecting subglottic pathology. If such pathology is suspected, radiography and endoscopy are usually required for its detection.
Radiography
Radiographic imaging is very important in evaluation of the pediatric airway. While plain radiographs are usually the first and only radiographic studies ordered for the evaluation of children with stridor, their usefulness is controversial. Chest radiographs prior to general anesthesia to evaluate for major vascular and pulmonary changes are justified. But correlation with plain radiographs and direct laryngoscopy and bronchoscopy has failed to show a strong correlation in many studies.
Airway fluoroscopy is a quick, noninvasive, and dynamic study of the entire airway that provides important additional information to the history and physical examination. It is far superior to plain films. Airway fluoroscopy has had a significant role in evaluating foreign bodies. Blazer et al, found that fluoroscopy contributed to the diagnosis in 80-90% of bronchial foreign bodies but only 32-40% of foreign bodies above the carina. As most foreign bodies are radiolucent, the advantage is that focal air trapping can be detected during dynamic movement of the airway.
Airway fluoroscopy is less sensitive to supraglottic and glottic lesions detecting only 33% and 17%, respectively, but is much more sensitive in detecting subglottic, tracheal and bronchial lesions, detecting 80%, 73%, and 80%. The main disadvantage of airway fluoroscopy is increased radiation exposure. To our knowledge, there is no literature to support an increased risk of malignancy after airway fluoroscopy, which is usually less than 1 minute and equivalent to 10 rads (0.1Gy).
Magnetic resonance imaging (MRI) with gadolinium or computed tomography (CT) with contrast is useful when the plain film or the clinical history suggests vascular compression or mass lesions of the mediastinum. Computed tomography is the most useful study to detect and delineate choanal atresia or stenosis when it is suspected. The barium esophogram or the modified barium swallow study can be useful when feeding difficulties and aspiration are associated with respiratory distress.
Treatment
The ultimate treatment outcome of a pediatric airway emergency is rapid reversal of the impending airway complication. If rapid reversal to the physiologic state is not possible, then temporary securing of the airway is the successful treatment outcome. The approach to management of children with any airway emergency should always include a full and frank discussion of the risks with the parents, and child if appropriate. The possibility of tracheostomy and failure to secure the airway should always be mentioned.
When a patient is unable to adequately perform self ventilation the most common method of securing the airway is oral or nasal intubation. In a large multicenter trail, pediatric intubations occurred in 156 of 1288 total emergency room intubations. Initial intubation attempts were all oral, and included rapid sequence intubation in 81%, without medications in 13%, and sedation with neuromuscular blockade in 6%. Older children and trauma patients were more likely to be intubated with RSI. Overall, successful intubation occurred in 99% of RSI and 97% of non-RS intubation attempts. Only one of 156 patients required surgical airway management. The Pediatric Emergency Medicine Committee of the American College of Emergency Physicians advocates consideration of RSI in every emergency intubation involving a child with intact upper-airway reflexes.
The use of helium-oxygen (Heliox) has been shown to be effective in cases of upper airway obstruction, postextubation stridor, and viral croup. The principle behind using heliox in these clinical situations is established by Graham’s Law, which defines the flow rate of any gas as being inversely proportional to the square root of the density. Helium is seven times less dense than nitrogen, which is the most abundant atmospheric gas. Because of its much lower density, replacing nitrogen with helium lowers airway resistance and decreases the work of breathing and alleviates airway obstruction. Patient selection for this treatment is widespread. In a study by Gosz et al, the majority of the diagnosis receiving heliox included congenital heart disease, laryngotracheobronchitis, prematurity and noncardiac postintubation. They found an immediated positive response, as measured by immediate decreased work of breathing within 5-10 minutes, in 73% of the patients. The average duration of treatment was 15minutes to 384 hours, with an overall mean of 29.1 hours. They found that patients with laryngotracheobronchitis were more likely to respond to heliox whereas the other causes were not statistically associated with response or failure.
It is preferable to perform a tracheotomy on an adult or a child in a controlled fashion. This, unfortunately, is not always the case. In a child, it is especially difficult to perform a cricothyrotomy because of the very small size of the membrane and difficulty in palpating thestructures. Some authors have suggested using a large-bore needle transtracheally, but this too is very difficult due to the flexibility of the child’s larynx and trachea. Basically, in a life-threatening situation, any means of obtaining an airway is better than the consequence of not. Complications of tracheostomy may be classified as early or late. Early complications include pnuemomediastinum/pnuemothorax, acute hemorrhage, accidental decannulation, tubal obstruction, and local infection. Late complications include tracheal granuloma, decannulation, subglottic stenosis, tracheocutaneous fistula
Prenatally detected giant neck masses such as cervical teratomas and cystic hygromas, and congenital high airway obstruction syndrome (CHAOS) caused by conditions such as laryngeal atresia can cause airway obstruction and may result in profound hypoxia or death due to the inability to obtain an airway at the time of birth. The EXIT (ex utero intrapartum treatment) procedure was originally described in cases of severe diaphragmatic hernia, it is now being used for other diagnoses which can obstruct the airway. In an EXIT procedure, the head, neck, thorax, and one arm of the baby are then delivered ensuring the umbilical cord is contained and not compressed within the uterus and the fetal airway is evaluated via bronchoscopy and intubation before the actual birth.
The utero-placental circulation and gas exchange is maintained during the procedure, and the surgeons are thus able to establish a fetal airway in preparation for birth. Occasionally, a tracheostomy or emergent resection of the mass is performed if necessary. Placental circulation allows surgeons 45 to 60 minutes to secure the airway, administer exogenous surfactant, and place umbilical lines. Additional surgery may be indicated once the baby is born.
Etiologies of Airway Emergencies:
Neck Masses
Teratoid and Dermoid cysts are rare causes of neck masses. These developmental anomalies are composed of different germ cell layers. They are thought to possibly arise from isolation of pleuripotent stem cells during migration with resultant disorganized growth or from entrapment of germ cell layers at points of failed embryonic fusion lines. The lesions are classified according to their composition. Dermoid cysts are composed of mesoderm and ectoderm and may contain hair follicles, sebaceous glands, and sweat glands. They are often midline or paramedian, painless, and do not elevate with tongue protrusion.
Teratoid cysts and Teratomas contain all three germ layers. These lesions commonly present within the first year of life and are usually larger midline or paramedian masses than Dermoid cysts. Teratomas are distinguished by cellular differentiation enough to have recognizable organs or structures. The larger size of these lesions results in secondary aerodigestive compressive symptoms. There is an associated 20% incidence of maternal polyhydramnios which can often lead to the diagnosis with maternal ultrasound.
Lymphangiomas are lymphatic cysts that are isolated from their normal route of drainage into the venous system. The embryological development of the lymphatic system is theorized by the centrifugal theory and the centripetal theory. The centrifugal theory states that lymphatic channels grow outward from venous channels. The centripetal theory states that lymphatic channels grow independently of venous channels. Regardless of the theory, the lymphatic cysts become either totally or partially isolated from the venous system. Histologically, they are classified into capillary, cavernous, and cystic types based on the size of the lymphatic spaces.
Cystic hygromas are large, soft, painless, and compressible masses that usually present by the age of 3. These lesions most commonly present in the posterior triangle where mass effect is limited. When they present in the anterior triangles, airway obstruction is more of a concern. CT is of greatest diagnostic value. Spontaneous regression is rare and surgical excision for obstructing lesions is the treatment of choice. Recurrence rates are generally high because of the poor encapsulation and dissection planes.
The larger size and obstructive potential of lymphangiomas and teratomas may lead to CHAOS (Congenital High Airway Obstruction Syndrome). Emergent airway management at the time of delivery is key for survival. High resolution fetal ultrasound may show flattened diaphragms, polyhydramnios, and a cervical mass. Appropriate team members include a maternal-fetal medicine specialist, neonatologist, anesthesiologist and an otolaryngologist. Management involves elective Caeserean section with establishment of the airway while still on placental oxygenation.
Laryngotracheobronchitis
Infectious agents are frequent causes of airway emergencies in children. Viral laryngotracheobronchitis, known as “croup,” is the most common infectious cause of strider with a peak incidence in the second year of life. Parainflueza type 1 is the most common culprit. Croup usually develops from an upper respiratory infection (URI) to a “barking” cough and high-pitched inspiratory stridor associated with varying amounts of respiratory distress. Although radiography is not required in the presence of these classic symptoms, neck films often show the “steeple sign,” – symmetric narrowing of the subglottic space.
Treatments for croup include, humidification, inhaled and systemic steroids, and inhaled epinephrine. There is no scientific data for the use of a humidifier as air is 100% saturated by the time it reaches the nasopharyx during the inspiratory cycle. Racemic epinephrine provides vasoconstriction of the mucosa and bronchial smooth muscle relaxation. Because of the possibility of relapse and the short duration of action, any child that receives racemic epinephrine should be observed for at least 2 hours post treatment. The rationale for steroid use is very solid, however their effectiveness remains in question. It is important to remember that when utilizing systemic steroids it will take at least 3 hours for the medication t oproduce any significant effects. Thus far, nebulized budesonide rather than nebulized dexamethasone appears to be comparable to that of oral or intravenous steroids.
Children rarely require intubation for this disorder, and those who fail extubation or require repeated hospitalizations should undergo direct laryngoscopy and bronchoscopy to check for subglottic stenosis.
Bacterial Tracheitis
Membranous, or bacterial tracheitis is thought to be a complication of viral laryngotracheobronchitis. The most common causative agent is Staphylococcus aureus. Children with the disorder present after a URI that progresses rapidly to harsh biphasic stridor with high fever and respiratory distress. Treatment is by endoscopy (flexible or rigid) with suctioning of the bacterial membranes on the trachea, placement of an endotracheal tube and antibiotics directed against the most common etiologic agents. Extubation should not be attempted until the patient has remained afebrile and there has been much improvement in the secretions.
Acute supraglottitis
Acute supraglottitis is a disease that is caused by infection of the epiglottis and surrounding structures. The disease use to be caused frequently by Haemophilus influenzae type B in children 1 to 5 years of age. However, since conjugate vaccines for this bacteria were introduced in 1987, the disease’s incidence has declined greatly, the age of occurrence has increased, and other pathogens such as Candida albicans, Haemophilus parainfluenzae, and staphylococcus have become more common culprits. The child with the disorder typically presents with a mild URI that progresses over the course of hours to severe throat pain, drooling, fever and respiratory distress. The diagnosis is generally made by history with a very limited physical exam to avoid compromising the airway. Radiography is not generally necessary, but it will show epiglottic swelling, the “thumbprint sign.” Management is by halothane and oxygen induction of anesthesia, placement of an intravenous line, and direct laryngoscopy with blood and epiglottic cultures, followed by endotracheal intubation. Extubation is carried out when leak around the endotracheal tube occurs with less than 20 cm H2O of pressure. Antibiotic therapy with ampicillin and chloramphenicol are initiated and modified to culture results.
Congenital Syndromes
It is not surprising that patients with craniofacial syndromes frequently have associated airway anomalies because of the close embryologic development of the airway with the craniofacial structures. Prominent abnormalities may become immediately apparent at birth or soon after, or they may be more subtle take months or years to develop. Also, the etiologies of craniofacial syndromal airway obstruction and restriction are not based only on anatomic deformity, such as in the case of mucopolysaccharidoses, in which there is infiltraton of the soft tissues of the airway.
Pierre Robin sequence consists of micrognathia and relative macroglossia with or without cleft palate. In the severe case, airway obstruction develops in the first four weeks of life. Especially when the baby is supine, the nasopharynx will fill with the tongue and cause varying degrees of nasal obstruction. Matters become worse when the child becomes agitated and generates greater negative thoracic pressures. The larynx can almost appear invisible to conventional equipement and is best approached by sweeping the tongue in order to visual the laryngeal structures.
Treacher Collins syndrome consists of abnormalities of the external, middle and inner ear, mandibule, and minor eye abnormalities. Usually the zygoma and mandible are hypoplastic. The degree of hypoplasia may render the larynx nearly impossible to approach via the midline. Goldenhar syndrome may present with similar maxillomandibular findings as Treacher Collins but may also be complicated by a short immobile neck.
The mucopolysaccharidoses (Hurler’s, Hunter’s and Marateaux-Lamy symdromes) are hereditary progressive disorders in which deficiency of an enzyme results in excessive intralysosomal accumulation of mucopolysaccharidoses. This causes a generalized infiltration of the structures of the airway. The tongue may fill the oropharynx, the neck is typically short and immobile and the temporomandibular joints may be involved.
Children with Down’s syndrome have midface hypoplasia, macroglossia, a narrow nasopharynx, and a shortened palate. These anatomical abnormalities along with generalized hypotonia, an immature immune system, and a tendency towards obesity predispose them to upper airway obstruction. Furthermore, gastroesophageal reflux disease is very common in these children and may
Nasal obstruction
Nasal obstruction is typically an airway emergency only in the neonate, since newborns are obligate nasal breathers. Causes of nasal obstruction in neonates and infants are varied. Choanal atresia should be suspected in an infant whose nasal airways are shown to be occluded by methodologies suggested above. These infants often have severe respiratory distress alleviated somewhat by crying. Computed tomography confirms the diagnosis. Choanal atresia usually presents as an airway emergency only when it is bilateral. Choanal atresia ocurrs in 1 in 8000 births with twice as high an incidence in female as males. It is believed to be caused by a failure of the breakdown of the bucopharyngeal membrane or persistence of epithelial rest cells in the nasal cavities during embryonic development. The atresia usually has mixed bony and membranous components. Choanal atresia can present as part of the CHARGE association, consisting of colobomas, heart abnormalities, renal anomalies, genital defects and ear (external, middle and inner) anomalies. Diagnosis of choanal atresia should prompt a search for those associated anomalies. Bilateral choanal atresia is managed urgently with a taped-in neonatal oropharyngeal airway or a McGovern nipple and nasogastric feeding. Definitive surgery is usually carried out soon after diagnosis, though it can be delayed in the presence of other pathology.
Laryngomalacia
Laryngolmalacia is the most common congenital laryngeal anomaly. The incidence among males is twice that among females. The symptoms often do not present for days to weeks after birth, but then peek at around two weeks after birth and resolve by 12 to 18 months. The disorder is associated with a low pitched stridor that has a fluttering quality and is more noticeable when the child is agitated or in the supine position. The stridor is relieved by placing the patient prone and extending the neck. On endoscopy, laryngomalacia is characterized by several anatomic abnormalities including shortened aryepiglottic folds and a curled epiglottis (“omega shaped epiglottis”). Shortened aryepiglottic folds can draw the cuneiform and corniculate cartiages forward over the laryngeal inlet, causing the larynx to prolapse during inspiration. Most theories about the cause of laryngomalacia center on the immaturity of the cartilaginous supraglottis and on immature laryngeal neuromuscular control. Surgical intervention usually is not necessary. However, for symptoms of obstructive apnea, failure to thrive, or cor pulmonale, supraglottoplasty is performed during direct laryngoscopy. This procedure is individualized to the infant’s anatomy contributing to the airway collapse. It can include trimming of the epiglottis, excision of redundant mucosa and cartilage in the cuneiform and corniculate cartilages, or removing a wedge of tissue from the aryepiglottic folds.
Subglottic Hemangioma
Subglottic hemangiomas are benign congenital vascular tumors characterized by cellular hyperplasia of endothelial cells, mast cells, fibroblasts, and macrophages. Subglottic hemangiomas grow rapidly, but growth generally slows in the first year of life and the hemangiomas generally resolve by the age of five. Their incidence is two times as common among females as males. Infants become symptomatic usually between the age of 3 to 6 months with inspiratory or biphasic stridor, barking cough and occasionally hoarseness. Only rarely does the disorder present as an airway emergency. Asymmetric subglottic narrowing is seen on anteroposterior neck films. MRI or CT demonstrate the extent of the lesion, including extension into the neck or mediastinum. Diagnosis by endoscopy is possible without the need for biopsy because the lesion has a characteristic appearance of a compressible asymmetric submucosal mass, occasionally with a bluish or reddish discoloration and in the posterior lateral subglottis. Choices for therapy are many and include tracheotomy (avoided if possible), systemic or intralesional steroids, the CO2 laser for partial excision, open surgical excision, and interferon alfa-2a therapy.
Laryngeal Web and Atresia
Laryngeal webs and atresias are caused by failure of recanalization of the larynx during prenatal development. Most of these disorders occur in the glottic area and have an extension into the subglottic larynx. Congenital webs rarely occur in the supraglottic larynx. Webs of the larynx only rarely present with airway emergency. Instead, they most commonly present with hoarseness in the cry or aphonia or stridor. Complete laryngeal atresia presents as a respiratory emergency that leads to death unless emergent tracheotomy is performed. Minor webbing with no airway symptoms can be observed, but more severe webs require tracheotomy, incision of the web with keel placement, and possible laryngeal reconstruction.
Tracheoesophageal Fistula and Laryngeal Cleft
Tracheoesophageal fistula takes many different forms and can present as respiratory distress, particularly in cases in which esophageal atresia is present, where children present with aspiration and cough. Laryngeal clefts are rare anomalies in which, like with tracheoesophageal fistula, the lumen between the larynx and esophagus fails to completely develop. Treatment for mild laryngeal clefting may include observation, but more severe forms, and all forms of tracheoesophageal fistula require operative repair.
Vascular Anomaly
Vascular anomalies may lead to significant respiratory distress in the neonate because of the proximity of the great vessels to the airway. These disorders are best diagnosed by MRI or CT with contrast when suspected. An aberrant right subclavian artery sometimes arises from the left descending aorta, passes behind the esophagus and trachea and then follows its normal course on the right side. This produces aspiration and respiratory distress, and is strongly suggested by compression on a barium esophagram. The double aortic arch malformation is characterized by duplicate aortic arches that meet posterior to the esophagus and trachea and lead to formation of a complete vascular ring. Treatment is by division of one of the arches. Inominate artery compression of the trachea can lead to apneic episodes and respiratory distress when the artery arises more to the midline than usual.
Airway Foreign Bodies
Airway foreign bodies are common causes of respiratory distress in children. The problem includes foreign-body aspiration, in which the object is lodged in the laryngotracheobronchial axis, and foreign-body ingestion, in which the object is found in the esophagus. The most common age group affected is children between 2 and 4 years of age, with a male to female ratio of 2:1. Most often, children have an acute episode of choking and gagging, though if this episode is not witnessed, diagnosis by history may be difficult. Patients may present with nonspecific pulmonary complaints such as intermittent coughing or wheezing; or they may present with severe respiratory distress. The most common culprits are food products, such as nuts, raisins, or seeds. Some objects, such as seeds, will swell and lead to complete bronchial obstruction. Plastic can be nonradiopaque and undetectable on plain films. Expiratory chest film is most useful in evaluation of foreign-bodies. Fluoroscopy may be necessary in younger children to obtain good expiratory views. On expiration, mediastinal shift and air trapping is seen. Bronchoscopy should be performed in the presence of suspicious history even in patients with negative films because of the risk of false negative films in up to 25% of patients. Rigid bronchoscopy allows removal of the foreign body and should be followed by careful examination of the airway for distant lesions. Post-instrumentation edema may necessitate intravenous steroids and inhaled bronchodilators. Esophageal foreign bodies may present with respiratory distress due to compression of the posterior tracheal airway, especially in smaller children. This occurs most commonly when the foreign body lodges at the level of cricopharyngeal muscle. Esophageal foreign bodies should be treated as airway emergencies because of the risk of conversion of an upper esophageal foreign body into an airway foreign body.
Direct laryngoscopy and bronchoscopy is most safely and efficiently accomplished by a ventilating bronchoscope under general anesthesia. The procedure should be carried out sooner than later, considering other patient factors, to decrease the hazard of an impending airway complication, such as an esophageal foreign body migrating into an airway foreign body, or swelling of an organic object.
Conclusion
Airway management and endotracheal intubation in children requires a solid knowledge of the anatomical differences found in the pediatric upper airway. Early assessment of the urgency of the situation followed by proper planning and airway team assembly will increase the likelihood of a successful outcome.
Subglottic Stenosis
Anatomy
The subglottis is defined as the area extending from the lower surface of the true vocal cords to the lower surface of the cricoid cartilage. In adults this corresponds to approximately 10 mm inferior to the anterior commissure and 5 mm inferior to the posterior commissure.
The infant larynx differs significantly in size and position when compared to the adult larynx. At birth, the infant larynx is approximately one third the size of the adult larynx, however, the infant larynx is proportionately larger than the adult larynx compared with the remainder of the tracheobronchial system. The vocal process of the arytenoid takes up half the length of the vocal cord in the infant larynx, while it only takes up about ¼ of the length of the vocal cord in the adult. The narrowest portion of the airway in the older child and adult is the glottic aperture, while the narrowest part of the airway in the infant is the subglottis. The subglottis in infants measures approximately 4.5 by 7mm. A diameter of 4.0 mm is considered the lower limit of normal in a full term infant and 3.5 mm in a premature infant. One millimeter of edema circumferentially in the subglottis reduces the cross-sectional area by 60%.
The infant larynx is positioned higher in the neck than the adult larynx. The superior border of the larynx of the infant is located at about the level of the first cervical vertebrae with the cricoid positioned at about the fourth cervical vertebrae. In comparison, the adult cricoid rests at about the level of the sixth cervical vertebrae. The structures of the infant larynx are more pliable and less fibrous making the infant airway more susceptible to narrowing from edema and less easily palpable.
Embryology
The respiratory system is an outgrowth of the primitive pharynx. The development of the lower respiratory system begins at 26 days after conception as the laryngotracheal groove at the ventral aspect of the foregut forms.
The laryngotracheal diverticulum becomes separated from the foregut by the tracheoesophageal folds which fuse to become the tracheoesophageal septum. This septum divides the foregut into a ventral laryngotracheal tube and a dorsal esophagus. Failure of the tracheoesophageal folds to fuse during the fourth and fifth weeks can lead to a tracheoesophageal fistula.
The larynx develops from the fourth and fifth branchial arches. The laryngotracheal opening lies between these two arches. This primitive laryngeal aditus is altered to become a T-shaped opening by the growth of three tissue masses. One is the hypobranchial eminence. This mesodermal structure eventually becomes the epiglottis. The second and third growths are two arytenoid masses. As these masses grow between the fifth and seventh weeks, the laryngeal lumen is obliterated. Recanalization occurs by the tenth week. Failure to recanalize may result in atresia, stenosis, or web formation in the larynx. The arytenoid masses are separated by an interarytenoid notch which eventually becomes obliterated. If obliteration does not occur, a posterior laryngeal cleft can result leading to severe aspiration in the newborn
Etiology of SGS
- Congenital SGS
- Membranous
- Increased fibrous connective tissue
- Hyperplastic submucous glands
- Granulation tissue
- Cartilagenous
- Cricoid cartilage deformity
- Small cricoid
- Elliptical cricoid
- Large anterior lamina
- Large posterior lamina
- generalized thickening
- Submucous cleft
- trapped first tracheal ring
- Acquired SGS
- Intubation
- Laryngeal trauma
- previous airway surgery
- High tracheostomy
- Cricothyroidotomy
- prior surgery for respiratory papillomatosis
- prior laser surgery for SGS
- accidental
- Inhalational (thermal or caustic)
- trauma (blunt or penetrating)
- Autoimmune
- Infection
- Gastroesophageal reflux (GER)
- Inflammatory diseases
- Anti-neutrophil Cytoplasmic Autoantibodies (C-ANCA)
- Sarcoidosis
- Systemic lupus erythematosis
- Neoplasms
- Idiopathic SGS
Congenital SGS
SGS may be classified as either acquired or congenital. Although congenital subglottic stenosis is uncommon, accounting for 5% of all cases, it is the third most common congenital airway problem (after laryngomalacia and vocal cord paralysis). Congenital SGS is thought to be secondary to failure of the laryngeal lumen to recanalize properly during embryogenesis. SGS is considered congenital if there is no history of endotracheal intubation or other forms of laryngeal trauma.
Congenital SGS is divided histopathologically into membranous and cartilaginous types. Membranous SGS is usually circumferential and consists of fibrous soft-tissue thickening caused by increased fibrous connective tissue or hyperplastic submucous glands. It may involve the vocal folds as well. The cartilaginous type usually results from a thickened or deformed cricoid cartilage that forms an anterior subglottic shelf that extends posteriorly allowing only a small posterior opening. Other malformations can occur such as an elliptical cricoid leaving a slit-like opening or a trapped first tracheal ring. Membranous SGS is usually less severe than the cartilaginous type.
Congenital SGS is often associated with other congenital malformations. A thorough search for associated anomalies is necessary.
Acquired SGS
Ninety-five percent of cases of SGS are acquired and may be due to a number of causes. The most common cause of acquired SGS (90%) results from endotracheal tube intubation and
the associated inflammatory-type response (see below – pathology). The most important risk factor for the development of laryngotracheal stenosis is the duration of intubation. Other factors include size of the endotracheal tube, movement of the endotracheal tube, traumatic intubation, number of re-intubations, and presence of an infection while intubated.
Other causes of acquired SGS include external and post-surgical trauma, and systemic factors. Laryngotracheal injuries from blunt trauma may result in fractures, dislocations, mucosal lacerations, or cricotracheal separation. Wound healing in these instances occurs as described below with development of granulation tissue and then deposition of fibrous tissue and subsequent scarring which may result in clinically apparent stenosis. Caustic and thermal injuries also cause mucosal injury, ischemia and subsequent remodeling of tissues and may result in LTS.
Post-surgical trauma includes previous tracheotomy (particularly high tracheotomy), cricothyroidotomy, and surgical treatment of airway neoplasms (most commonly recurrent respiratory papillomas and subglottic hemangiomas). The incidence of subglottic stenosis has been reported to be as high as 20% in patient undergoing laser excision of subglottic hemangiomas (Sie, 1994).
Gastroesophageal reflux (GER) has been proposed as a medical condition which may exacerbate the pathogenesis of LTS, may cause re-stenosis after repair, and may be the sole cause of stenosis in patients with no previous history of endotracheal intubation or laryngotracheal trauma. Bain et al. (1983) was the first to suggest that GER may cause SGS. He identified GER in two patients with SGS. Since then animal studies have been performed which have shown that superficial mucosal injury in the trachea and subglottis treated with acid and acid with pepsin result in non healing and inflammation (Koufman, 1991) and may lead to SGS (Little, 1985). Little’s study, however, had only one subject in each group and therefore significant results were not obtained. Other studies have shown a high rate of GER in patients with SGS. Walner et al. (1998) restropectively looked at 74 pediatric patients with SGS and found that they had a three times greater incidence of GER than the general pediatric population. They were unable to draw any conclusions about the relationship between GER and SGS because of the diversity of their patient group. Koufman (1991) found that 72% of 32 patients (11 pediatric and 21 adult) with laryngeal and tracheal stenosis had abnormal lower pH probe results. 67% of those patients had abnormal pharyngeal reflux defined as a single episode of a pH less than 4.0.
The current criteria for diagnosing lower esophageal acid reflux is well documented (a pH of less than 4.0 for more than 10% of the time), however, despite the fact that pH-metry is considered the gold standard for LPR testing, there is no consensus with respect to the number of pH sensors, their location, or the interpretation of results in adults or children (Postma, 2002). Postma suggests four criteria that must be met for an event to be defined as a pharyngeal reflux episode:
- A decrease in the pH level to less than 4.0 (may be increased to 5.0)
- A decrease in the pharyngeal pH level immediately following distal esophageal acid exposure
- No decrease in the pH level during eating or swallowing
- A rapid and sharp decrease in the proximal sensor pH level rather than a gradual one
Pepsin is functional at a pH of 5.0. The laryngopharynx is much more susceptible to the inflammatory affects of acid and pepsin because it lacks the protective mechanisms that the stomach and lower esophagus have. As was shown in Koufman’s study (1991), nonhealing ulcers and inflammation resulted in areas of damage to the subglottis as a result of acid and pepsin administration. It is logical to think that exposure to a single episode of LPR in patients who have existing laryngeal or subglottic injury may have significant effects.
Despite the lack of definitive evidence for a cause and effect relationship between GER and SGS, Cotton and O’Connor state that a “reflux workup” is now considered essential to the success of laryngotracheal reconstruction (LTR) (1995). Empirical treatment of GER has been recommended for all patients undergoing LTR (Burton, 1997) even if they do not have symptoms of GER or LPR or documented reflux by pH probe.
Idiopathic subglottic stenosis
Pathology
The pathogenesis of acquired subglottic stenosis is not completely understood but there are several theories that have been proposed. The most accepted theory proposes that subglottic stenosis results from wound healing in the areas of the airway which have undergone compression by an endotracheal tube or the cuff of a tube resulting in necrosis of the underlying mucosa and cartilage. This necrosis is a consequence of ischemia resulting from pressure from the tube or cuff exceeding the capillary pressure of the thin mucosa of the airway. Consequently, the normal mucociliary flow is disrupted which leads to infection in the perichondrium and then extends into cartilage. The cartilage may weaken and collapse, manifesting as tracheomalacia. Healing of the involved segment proceeds by secondary intention. This involves three temporally overlapping stages: an inflammatory stage, a proliferative stage, and a phase of contraction and remodeling. The inflammatory stage begins with the initial injury. It involves active vascular retraction and constriction followed by vasodilation mediated by prostaglandins. Platelets adhere to the exposed collagen forming a hemostatic plug. There are numerous active mediators that are released including adenosine diphosphate, platelet derived growth factor, transforming growth factor, clotting factors and vasoactive substances such as 5-hydroxytryptamine, thromboxane and histamine. The coagulation cascade is activated which lays down the fibrin network. Platelets release proteolytic enzymes that activate the complement system. The complement cascade contributes to bacterial killing and wound debridement and supplies chemotactic signals to effect cells.
Polymorphonuclear lymphocytes (PMNs) enter the wound at about 6 hrs post-injury. They reach the their greatest concentration at 24-48hrs post-injury and disappear within 72hrs. The PMNs phagocytize debris and bacteria from the wound and release proteases that lyse devitalized tissue.
T and B cells enter the wound after the PMNs and are most numerous at 6 days after injury. T cells secrete regulatory lymphokines. Helper T-cells are necessary for wound healing. Suppressor T cells down-regulate healing.
Macrophages enter the wound within 48-96 hrs after injury. Large concentrations of macrophages are found in the wound at 3-5 days and can persist for several weeks. Macrophages are the only cells that can function in area of low oxygen tension at the wound center. These cells release many different enzymes but they primarily direct the proliferative and synthetic activity in the wound. This is achieved by interleukin-1 and tumor necrosis factor –alpha. Studies have shown that macrophages are essential to wound healing but PMNs can be absent without impairing the process.
Growth factors secreted by cells in the wound are major regulators of healing. Growth factors are chemotactic, mitogenic, and sometimes have an inhibitory effect on cells involved with wound repair. All growth factors interact with cellular receptors to modify the cells activities. IL-1 directly stimulates fibroblast activity including proliferation and collagen synthesis. IL-2 is produced by helper T cells. It has an indirect effect on wound healing by stimulating lymphocyte and macrophage activity.
Structural components essential to successful wound healing include fibronectin, collagens, glycoproteins and glycosaminoglycans. Fibronectin is secreted by fibroblasts and endothelial cells. It cross-links fibrin and glycosaminoglycans. Several types of collagen are found in the healing wound. The production of collagen by fibroblasts is a complex process. Hydroxylation requires Vitamin B, iron and Vitamin C. Glycoproteins and glycosaminoglycans function in cell-matrix interactions and in controlling wound resilience.
The proliferative phase lasts 10-14 days. It begins with re-epithelization. This process of resurfacing the defect begins at about 12 hrs after injury. The epithelial cells 1-2 mm from the wound edge undergo phenotypic changes. The cells replication rate increase 17 fold.
The next part of the proliferative phase is neovascularization in which blood vessel regeneration begins with the migration of endothelial cells. Macrophages secrete angiogenic factors in response to high lactate levels and low wound oxygen levels. The result of endothelial migration is capillary bud formation. Certain conditions such as diabetes mellitus or radiation therapy impede neovascularization.
Collagen deposition begins when fibroblasts enter the wound at 48-72 hrs. post-injury. The collection of fibroblasts, inflammatory cells and capillary buds is referred to as granulation tissue. Granulation tissue persists until epithelial resurfacing is complete.
The final phase of wound healing is the wound contraction and remodeling phase. Wound contraction begins at 6-7 days after injury and is maximal for 10 days. Wound contraction eventually decreases the defect by 40-60%. Skin flaps and grafts can reduce the amount of wound contraction by 50-70%. Myofibroblasts, a modified fibroblast, provides the contractile force required for contraction. They have many characteristics of smooth muscle cells and are distributed throughout the wound. Wound collagen levels reach maximum at 2-3
weeks after injury, however, tissue strength is still only 5-10% of that of unwounded skin. The wound’s neo-matrix is gradually replaced over 6-12 months by stronger interwoven cartilage as Type I collagen displaces Type III cartilage. Remodeling results in a scar with as much as 80% of the involved tissue’s original tensile strength.
Diagnosis
There is a wide range of presentation of subglottic stenosis with similarities and differences in the pediatric age group compared to adults. If the stenosis is severe and congenital, the patient will present with airway distress at birth. More commonly, the pediatric patient with SGS with present as a neonate in the intensive care unit who has failed extubation usually multiple times.
Occasionally patients will present with in clinic with a tracheotomy and the report of some airway obstruction. Infants with mild SGS may present with recurrent croup-like illnesses and poor feeding. Adults usually present with a history of prior intubation with symptoms of progressive shortness of breath and noising breathing.
In the infant or child, a thorough history should be obtained which includes: history of prematurity and associated medical problems and intubation records. A history of noisy breathing and difficulty feeding should lead to suspicion of airway problems. Growth curves should be reviewed and followed to determine if the child has failure to thrive. Particularly the relationship of airway symptoms to feeding is important to elicit in the history. Important characteristics of the intubation include the date of first intubation, duration, size of the endotracheal tube, number of intubations, and if any intubations were traumatic.
In adults, intubation records should be reviewed as well. A complete medical history is taken with particular attention to the patients cardiopulmonary status, history of diabetes, and any steroid use. History of reflux symptoms should be elicited which may prompt subsequent testing.
Differential diagnosis of laryngotracheal stenosisI. Congenital
- Tracheomalacia
- Laryngomalacia
- Vocal cord paralysis
- Laryngeal cleft
- Congenital cysts
- External compression from congenital abnormality or lesion
- Vascular compression
- innominate artery compression (most common)
- right-sided aortic arch with persistent ductus arteriosus
- aberrant left pulmonary atery
- Mass
- teratoma
- cystic hygroma
- hemangioma
- Infectious/inflammatory
- Viral laryngotracheobronchitis (croup)
- Retropharyngeal abscess
- GER
- Tracheitis
- Neoplastic
- Subglottic hemangioma
- Recurrent respiratory papillomatosis
- Traumatic
External compression
Foreign body
A complete head and neck exam should be performed on all patients. It begins with observing the patient for any apparent airway symptoms which may include irritability and restlessness in an infant or dyspnea, tachypnea, cyanosis, and stridor in an infant, child, or adult. The classic stridor of subglottic stenosis is biphasic. Voice quality in a child or adult and crying quality in an infant should be evaluated for weakness, hoarseness, breathiness, or complete absence. Flexible fiberoptic nasopharyngolaryngoscopy may be performed at the bedside or in clinic on infants, adults, and some cooperative children if the patient is stable. Vocal cord immobility, reflux changes, immediate subglottic abnormalities, supraglottic sensation, and other glottic and supraglottic abnormalities may be detected.
The gold standard for diagnosis of any laryngotracheal abnormalities is direct laryngoscopy and tracheobronchoscopy under general anesthesia. This should be performed in the operating room with an experienced anesthesiologist. It is important to delay endoscopy for at least two weeks following an acute episode of croup to minimize the risk of postoperative airway obstruction. The potential need for tracheotomy should be discussed with the patient (adults) or patient’s family (children) prior to endoscopy. A rigid bronchoscope or a rod lens telescope may be used to assess the airway. The important things to document during endoscopy are as follows: (1) the outer diameter of the largest bronchoscope or endotracheal tube that can be passed through the stenotic segment, (2) the location/subsites (glottis, subglottis, trachea) and length of the stenosis, (3) other separate sites of stenosis, (4) other airway anomalies in infants (clefts, webs, cricoarytenoid joint fixation, neoplasms, etc.), and (5) reflux changes. After removing the sizing endotracheal tube or bronchoscope it is important to observe the stenotic segment for edema which may result in the need for tracheostomy
There are two widely excepted staging systems for classifying subglottic stenosis: Myer-Cotton grading system and the McCaffrey system. Other systems have been described as well, however, none are universally applicable or useful. At this time, no staging system exists that allows comparison of patients treated at different institutions.
The Myer-Cotton staging system is useful for mature, firm, circumferential stenosis confined to the subglottis. It describes the stenosis based on the percent relative reduction in cross-sectional area of the subglottis which is determined by differing sized endotracheal tubes.
Four grades of stenosis are described with this system: grade I lesions have less than 50% obstruction, grade II lesions have 51% to 70% obstruction, grade III lesions have 71% to 99% obstruction, and grade IV lesions have no detectable lumen or complete stenosis.
The McCaffrey system classifies laryngotracheal stenosis based on the subsites involved and the length of the stenosis. Four stages are described: stage I lesions are confined to the subglottis or trachea and are less than 1cm long, stage II lesions are isolated to the subglottis and are greater then 1 cm long, stage III are subglottic/tracheal lesions not involving the glottis, and stage IV lesions involve the glottis.
Another classification system has been proposed by Lano et al. at Vanderbilt University (1998) for adults. There system is based on the number of subsites (including the glottis, subglottis and trachea) of involvement: stage I lesions involve one subsite, stage II involves two subsites and stage III involves all three subsites. The authors proposed this classification to better predict patient prognosis (successful decannulation) after reviewing their experience over ten years.
Radiographic tests that may be ordered to evaluate the patient with upper airway problems include plain films, barium esophagram, airway fluoroscopy, magnetic resonance imaging (MRI), and computed tomography (CT). Plain films are more useful in the pediatric population and may be used to diagnosis foreign bodies, narrowing of the subglottis and trachea in croup, epiglottis, retropharyngeal abscess, subglottic cystes, and subglottic hemangioma. They are cheap, easily accessible, and can be done without sedation. If adequate inspiratory and expiratory films cannot be obtained, fluoroscopy may be used. Airway fluoroscopy is dynamic and can show areas of malacia. Barium swallow studies are not useful specifically in the diagnosis of SGS but are indicated when assessing feeding problems in infants and can be used to rule out other anatomic abnormalities such as esophageal compression from vascular anomalies, esophageal masses and may show evidence of reflux. MRI is useful in diagnosing soft tissue masses. CT scanning with thin cuts (1.5) through a stenotic segment of the trachea and/or subglottis can help determine the length of resection that will be needed. CT has not been shown to be useful in diagnosing or characterizing SGS in the pediatric age group but is useful in adults as an adjunct to endoscopy. MRI and CT usually require sedation or general anesthesia in young children and infants.
Management
As previously mentioned, all patients must undergo a complete history, physical examination, flexible nasopharyngolaryngoscopy and +/- radiologic evaluation to make the diagnosis. This is followed by rigid endoscopy to evaluate the larynx, trachea, bronchi, and esophagus and to confirm the diagnosis and characterize the stenotic lesion. The management of the lesion will then depend on its diameter, length, location and the status of the patient.
Medical
Prior to airway reconstruction, it is recommended that all pediatric patients be evaluated for GER with a dual 24hr pH probe(Cotton and Walner, 1999). This is performed with the
consultation of a pediatric gastroenterologist. Different sized pH probes are used depending on the age of the child. Position of the probe should be confirmed with fluoroscopy. In unusual cases the refluxate may be basic in pH. In these patients, diagnosis is made with a nuclear medicine reflux scan. Patients diagnosed with GER should be treated accordingly. Adults are not always subjected to an extensive workup for GER unless symptoms are present. Empiric peri-operative treatment with anti-reflux medications has been recommended by some authors and is practiced by many.
In adults it is important to evaluate the patients general medical condition prior to performing any reconstructive procedures. Many of these patients underwent long periods of intubation secondary to severe medical problems. The decision to perform surgery should be made in consultation with the patient’s primary or specialty physician (pulmonary, cardiology, nephrology, etc.). Relative contraindications to LTR in adults are renal failure, diabetes, severe coronary artery disease, severe COPD or restrictive lung disease, obstructive sleep apnea, and systemic steroid use. It is important to consider each patient’s case on an individual basis and make the decision to proceed with surgery based on sound judgment.
Observation
Patients (children and adults) with Cotton-Myer grade I and mild grad II subglottic stenosis may sometimes be managed with close observation (Walner and Cotton,1999). In adults, this will depend on the reliability of the patient for close follow-up and their symptomatology. Children may be watched closely if they have only occasional mild stridor without retractions or feeding difficulties and have not required hospitalization for episodes of croup or other airway-related illnesses. Walner and Cotton recommend repeat endoscopy every three to six months to measure the diameter of the airway to ensure that it is enlarging as the child grows. As stated previously, the child should be followed with growth curves by a pediatrician and/or neonatologist.
Surgical treatment options for subglottic stenosis:
- Tracheostomy
- Endoscopic
- Dilation
- Endoscopic laser excision
- Open procedure
- Expansion procedure (one-stage or with stent placement)
- Anterior cricoid split with or without cartilage graft*
- Posterior cricoid split with or without cartilage graft*
- Anterior and posterior cricoid split with cartilage graft
- Four quadrant LTR
- Segmental resection (cricotracheal resection – CTR)
- Primary CTR
- Salvage CTR
- Extended CTR – CTR with and expansion procedure, arytenoid lateralization, or aytenoidectomy
* Note: some authors classify laryngotracheoplasty as a cricoid split procedures without cartilage grafting and laryngotracheal reconstruction as a procedure with grafting or segmental resection
Tracheostomy is usually required as the inital step, particularly in the pediatric age group with acquired SGS, in the surgical management of patients with SGS. Infants with congenital SGS may forgo tracheostomy as they are often treated with primary reconstruction or are observed. Tracheostomy is often not performed in infants with congenital SGS. These patients are either treated conservatively or undergo primary reconstruction and are left intubated for approximately one weekInfants with SGS are often premature or of low birth weight. A tracheotomy allows these patients time to grow before definitive surgical treatment is performed. Cotton and Walner recommend waiting until the infant is at least 10 kilograms before performing airway reconstruction. Delayed reconstruction will also allow for optimization of pulmonary function in patients with bronchopulmonary dysplasia. It is important to note that the mortality rate of laryngotracheal stenosis is primarily due to complications from the tracheostomy of which plugging and accidental decannulation are the most common. The reported mortality rate of tracheostomy in infants and children is 2% to 5% per child per year (Lesperance, 1996).
Tracheostomy may be required in adults if the patient presents in airway distress. More commonly, adults with acquired LTS have progressive symptoms over time and present early enough to allow time to plan for definitive airway reconstruction as the initial treatment with or without tracheostomy.
Once the decision to perform airway reconstruction has been decided upon, the surgeon must choose the most appropriate procedure to perform. The site, grade, and length of stenosis are the major factors in determining which surgical procedure will be used for reconstruction. The goal of all surgical procedures for LTS is to maintain vocal function and allow for early decannulation with subsequent unrestricted activity. Contraindications to reconstruction includes patients who would remain tracheotomy dependent even after adequate laryngotracheal expansion (i.e. patients with severe aspiration or BPD requiring a tracheostomy for pulmonary toilet), children with gastroesophageal incompetance resistant to surgical and medical management, and patients with an absolute contraindication to general anesthesia.
Endoscopic
Mild stenosis (Cotton-Myer grades I and II) can usually be treated with endoscopic techniques such as dilation or CO2 laser resection. Factors associated with failure of these endoscopic techniques include: previous attempts at endoscopic repair, circumferential scarring, loss of cartilaginous support, exposure of cartilage during laser excision leading to chondritis, severe bacterial infection, posterior inlet scarring with arytenoid fixation, combined laryngeal or tracheal stenosis or vertical scar length >1cm. Endoscopic dilation has had disappointing results,
however, reported success rates with endoscopic laser resection of Grade I and II stenosis range from 66-80%.
Grade III or IV stenoses usually require some form of open surgical procedure. Several techniques have been described.
Anterior Cricoid Split
The anterior cricoid split (ACS) procedure was originally described for a neonate who has had multiple failed extubations instead of performing a tracheostomy (Cotton and Seid, 1980). This procedure is also used for older infants and those who are have already been tracheotomized. Indications were later expanded to patients with congenital subglottic stenosis. The lesion responsive to this procedure is a mild anterior subglottic narrowing with extensive fibrosis but a normal cricoid. ACS may also be used to decompress subglottic cysts. Strict criteria for ACS have been established by Cotton and include: extubation failure on two occasions or more due to laryngeal pathology, weight >1500g, no assisted ventilation for 10 days prior to evaluation, O2 requirements <30%, no CHF for one month prior to evaluation, no acute respiratory tract infection, no antihypertensive medications ten days prior to evaluation. The procedure is performed after direct laryngoscopic and bronchoscopic confirmation of the diagnosis. All other airway pathology must be ruled-out.
A vertical midline incision is made through the cricoid cartilage and first two tracheal rings as well as the lower thyroid cartilage. This allows the cartilages to spring open and allow edematous mucosa to drain, increasing airway size. Prolene stay sutures are placed on either side of the cricoid cartilage and the skin is re-approximated after placement of a drain. The child is then left intubated, sedated and paralyzed in the ICU for 7-14 days. Cotton has guidelines for endotracheal tube sizes for stenting and for duration of stenting based on the infants weight.
Laryngotracheal Expansion Surgery
Laryngotracheal expansion surgery involves scar division with distraction of the edges by interposition of graft material (augmentation) to widen the airway lumen. It is important to avoid removing scar which results in a large surface area of denuded mucosa and leads to restenosis.
Cotton recommends augmenting the airway with grafts when the distraction of the laryngotracheal framework must be greater than approximately 3mm. There are several techniques depending on the location and severity of the stenosis. Laryngotracheoplasty can be performed with a tracheostomy and formal stenting or by using the endotracheal tube as a stent, the latter known as a single-stage LTP (SS-LTP). There are a several stents that can be used for LTS including: endotracheal tubes, Silastic sheet rolls, Montgomery T-tubes, and laryngeal stents. Laryngeal stents include: teflon stents [Aboulker stent (short or long), ETS Poirot, Paris], and silastic stents (Montgomery stents: Boston Medical Products, Boston. The primary consideration when deciding on the type of reconstruction and stent material is to provide a safe airway and adequate support for the graft. Success of LTR among, other things, is determined by the surgical procedure, including possible need for stenting; choice of type and length of stent; and duration of stenting. Choosing the appropriate method for stenting requires
considering consistency of stenosis, altered anatomy, size, location and stability of grafts when used for sugical repair and host tissue healing factors (Zalzal, 1988)
Autogenous costal cartilage is the material of choice for grafting. Many other materials have been used for grafting including auricular, hyoid and thyroid cartilage and bone. Cartilage has much less resorption over time compared to bone. Although bone provides good structural support, grafts in the airway do no bear a lot of stress or weight.
Anterior laryngofissure with anterior lumen augmentation
This technique is good for anterior subglottic stenosis or anterior tracheal wall collapse. The lesion should not involve the glottis. Other procedures should be considered if there the cricoid cartilage is deformed or weak. Anterior grafts are made considerabley larger and thicker than grafts placed posteriorly. The perichondrium is oriented to the luminal side to allow for epithelialization. The perichondrium is also a good barrier against infection. A large external flange is created to prevent the graft from prolapsing into the airway.
Laryngofissure with division of posterior cricoid lamina
This is indicated for patients with posterior subglottic stenosis, posterior glottic stenosis that extends to the glottis, complete or circumferential stenosis, or if there is significant cricoid deformity. Division of the anterior and posterior cricoid must be carried out for this procedure. If possible, one should avoid a complete laryngofissure to to avoid damaging the anterior commissure, however this is often needed for posterior glottic involvement for access. The posterior cricoid cartilage is incised in a manner that is vertically oriented to the cartilage to allow maximal purchase for the graft. The incision is extended superiorly to the interarytenoid area and inferiorly 5 to10 mm into the membranous trachea. The graft is elliptical in shape. It should not be too thick as it can cause swallowing difficulties and can lead to aspiration. The width of the graft is determined by the desired distraction of the cut edges of the incised posterior cricoid cartilage. 0.05 to 1.00 mm of distraction can be obtained for each year of age, up to 1 cm. It is sutured in place with absorbable suture on a small cutting needle. The knots should be buried so that they remain extraluminal to prevent development of granulation tissue. Long-term stenting is usually necessary (3-6 months).
Laryngofissure and division of posterior cricoid lamina with anterior and posterior grafts
This should be used for patients who have SGS similar to those above but with a significant amount of stenosis posteriorly such that grafting is necessary to maintain the adequate separation.
Once the grafts have been sutured into place in any of the above procedures, the decision must be made on whether it should be single or double-staged. Cotton and Walner (1999) recommend a double-staged procedure for patients with severe stenoses, history of reactive airway, or poor pulmonary function. This should also be considered at institutions with inadequate intensive care facilities. Double-stage procedure implies placement of stent above the tracheostomy tube instead of using an endotracheal tube as the stent (single-staged
procedure). Once this decision is made, the strap muscles are closed to provide blood supply to the outer surface of the anterior graft.
Segmental resection/Cricotracheal resection (CTR) with thyrotracheal anastomosis
The first CTR was performed by Conley in 1953 in a patient undergoing surgery for chondroma of the cricoid cartilage. It was later popularized by Ogura and powers (1964) as a technique for treatment of traumatic stenosis. In the 1970s it became the treatment of choice in adults with acquired subglottic stenosis from long term intubation. Until recently, surgeons were reluctant to perform this procedure in the pediatric patients because of the risk of anastamotic dehiscence and recurrent laryngeal nerve injury, and disturbing the normal growth of the larynx. The first successful CTR performed in a child was occurred in 1978 (Savary). It wasn’t until 1993, however, that the first series of 15 pediatric patients treated with CTR for severe LTS was published. Multiple subsequent series have reported using CTR for severe LTS with good outcomes (Monnier et al., 1995, Monnier et al., 1998, Stern and Cotton, 1999).
This technique is indicated if there is severe deformity of the cricoid making grafting very likely to fail. Most say that there must be at least 10 mm of normal airway below the glottis, however Cotton states that the resection can be up to the vocal folds but to expect prolonged edema. This technique is technically difficult due to the close proximity of the vocal cords and recurrent laryngeal nerves. Stenosis less than 4 cm can be resected by laryngeal release and cervical tracheal mobilization. Stenting is not required and the trachetomy tube can ususally be removed at around 4 weeks.
Post-operative Care
Patients undergoing open laryngeal surgery for SGS require post-operative care in an ICU that has an intensivist and staff familiar with the specialized care these patients require.
Since most of the children undergoing open laryngotracheoplasty have more severe SGS they have usually had a tracheotomy for a while. Children who undergo laryngotracheoplasty with stenting typically only require a few days of care in the hospital to be sure that the parents are educated about tracheotomy care and have all necessary equipment at home. Since they have a stent in place, they should be given antibiotics and antireflux medications should also be continued. The length of time a stent should be kept in place depends on what the airway requires. If stenting is needed only to hold a graft in place while healing, one week may be all that is needed. On the other hand if the stent is used to counteract scar formation and maintain the lumen, months of stenting may be required. Stents should be monitored ever 3 to 4 weeks for displacement and granulation tissue development. Once removed, the patient should undergo repeat endoscopy every 2 weeks until healing is complete at which time decannulation is considered.
Infants and children who undergo either ACS or one-stage LTP require more intense post-operative care in the ICU. They usually stay intubated for 7-14 days with the endotracheal tube acting as a stent. The patient requires heavy sedation with or without paralysis. The patients should be treated with broad-spectrum antibiotics and antiflux medications. Chest physiotherapy and log-rolling the patient every 4 hrs. is important to prevent atelectasis, pneumonia, and pressure sores. The majority of patients do not need continuous paralysis. The patient undergoes extubation when an air leak develops. An audible air leak at a pressure of 20cm H20 is a good prognostic indicator for successful extubation. Decadron (1mg/kg) is usually given 12 hours before extubation and for 5 days after.
Most children with LTS have documented GER prior to treatment and therefore are already on an antireflux medical regimen. This should be continued in the peri and postoperative period. Patients (children and adults) without symptoms of reflux prior to treatment and those with a negative reflux workup should be treated in the peri-operative period. Some authors suggest continuing treatment up to three months after surgery in theses patients.
Complications are infrequent but can include: atelectasis, pneumonia, malpositioned endotracheal tube, accidental extubation, occluded ET tube, wound infection, granulation tissue, restenosis, and tracheocutaneous fistula. Some complications related specifically to ACS and one-stage LTP that require prolonged intubation with sedation include narcotic withdrawal and transient skeletal muscle paralysis related to the prolonged muscle relaxation.
Outcomes
The goal of management of subglottic stenosis is decannulation. Success rates are dependent on the cause of the stenosis, the number of previous failed attempts, the status of the remainder of the airway, especially the glottis and the severity of the stenosis. Cotton has reported an overall pediatric LTR success rate of 92%, 97% for Grade II, 91% for Grade III, and 72% for Grade IV.
Bailey (1988) reported results of 131 pediatric airway reconstructive procedures. He had a 92% success rate with patients who underwent laryngotracheoplasty procedures (no grafting) and 80% success with patients who underwent LTR (with grafting). He did not report on use of any grading system.
Lano (1998) reviewed 41 cases of LTR in adults. He classified patient lesions with all three previously mentioned grading systems (Cotton-Myer, McCaffrey, and Lano). Over all 80% of patients were decannulated. He found that surgical outcome significantly correlated with the Lano and McCaffrey grading system, however, not with the Cotton-Myer system. He had 94%, 78%, and 20% success rates with Lano stage I, II, and III respectively. Interestingly, the best predictor of surgical outcome was obtained by multiplying the value from the Lano and the Cotton-Myer systems. Lano suggest that the reason for this is that his classification, which grades the extent of the lesion, multiplied by the Cotton-Myer system, which grades the severity of the lesion, provides a three-dimentional rating which is more characteristic of these lesions.
Lano’s study was the first to report on the success of LTR with respect to diabetes. 75% of patients with type I or II diabetes who underwent LTR failed decannulation. The most common cause of failure in these patients was exuberant granulation tissue and restenosis with thick scar formation. One had dehiscence of the anastomotic site and requirred a T-tube to
maintain an airway. Impairment of wound healing secondary to decreased microvascular blood flow and bacterial overgrowth probably account for these findings.
Monnier et al. (1999) has reviewed the experience with CTR at the Department of Otolaryngology at the University of Lausanne, Switzerland. 69 CTRs were performed (48 infants and children and 21 in adults). 95% and 100% of the pediatric and adult patients, all of whom had Cotton-Myer grade III or IV stenosis, were successfully decannulated . Stern and Cotton (1999) reported on 38 pediatric patients who underwent CTR for severe LTS (grade III and IV). 33 patients were successfully decannulated. Complications preventing decannulation in this study included one patient with persistent aspiration, three who restenosed, one with arytenoid prolapse, and one with recurrent layrngeal nerve injury. Overall, 94% successful decannulation has be reported in the literature when CTR is used in pediatric patients with severe LTS (Monnier, 1999).
Grillo (1992) has reported his experience with 80 CTRs. He reported that 97% of patients underwent successful decannulation. He further subdivided patients into groups depending on their voice quality and exercise tolerance. 22.5% had an excellent outcome defined as a normal voice and no activity restriction. 60% had a good outcome in which patients suffered only slight lessening of maximum volume of voice, slight hoarseness that did not impede vocal use, slight weakness of voice after prolonged use, diminished ability to sing, and adequate breathing for all normal activities. 10% had satisfactory results which were those with a hoarse voice and either slight wheezing or shortness of breath on exercise, not sufficient to impair usual activities.
Dysphonia following laryngotracheal reconstruction has not been extensively studied. Many authors report that a functional voice is restored in most patients. MacArthur et al. published a series of 12 patients (average age 6yrs) who underwent LTR. Most patients underwent both endoscopic evaluation and speech analysis. They found that 78% of the children had altered anatomy, 44% had altered function and 100% had decreased voice quality. They concluded that children with high grade subglottic stenosis and a history of multiple prior surgeries are at risk for a poor voice outcome after laryngotracheal reconstruction.
Prevention
Preventative measures used to reduce the likelihood of developing SGS are directed at the those events which are known or postulated to cause or exacerbate this problem. The endotracheal tube (ETT) is well established as the most common cause of SGS. As mentioned before, the size and movement of the tube, multiple and traumatic intubations, high cuff pressures, and duration of intubation all contribute to increase damage to the delicate mucosal lining of the airway. It is therefore important for the primary physician, anesthatist and/or intensivist to be aware of SGS as a complication of endotracheal intubation. This starts with education. From there, minimizing the amount of trauma to the airway is key.
Choosing the appropriate size ETT should be determined by the patients age and size. If unsure, a smaller ETT can be inserted and if the leak pressures are to low, a half size larger tube can then be placed. The ideal size tube in children allows an air leak at an inspiratory pressure of
20 cm H2O. Using this guideline, Cotencin and Narcy (1993) found a very low rate of post extubation stridor in neonates (5/247 patients) with none requiring surgical therapy for stenosis. In adults, a leak should be heard at a peak pressure of 20-25 cm H20. In addition, cuff pressure testing by a respiratory therapist should be routine in an intensive care setting. Pressure should be kept below 25 cm H2O.
Blind intubations should always be avoided if possible. If direct laryngoscopy is difficult, fiberoptic intubation should be attempted. Once a patient is intubated, multiple measures should be taken to prevent the tube from moving. These measures are usually carried out by an anesthesiologist or an intensive care unit respiratory therapist. It is particularly difficult to prevent tube movement in nonparalyzed patients with neurologic injuries or in children. Accidental extubations should be avoided at all costs as these often result in traumatic reintubations. The number reintubations can be minimized by appropriately evaluating the patient with weaning parameters and establishing the presence of an air leak.
There is no definitive safe period for intubation. 5 to 10 days of intubation is generally acceptable in adults after which time a tracheotomy should be considered. Premature infants have much more pliable cartilages allowing for longer periods of intubation. Lesperance and Zalzal (1996) recommend performing a tracheotomy in premature infants after 50 days of intubation.
Most patients who are intubated in the intensive care unit receive intravenous antireflux medications. This is important because these patients are predisposed to reflux. Nasogastric tubes act as a stent and allow gastric contents to breach the upper and lower esophageal sphincters. If possible, the head of bed should be elevated to allow gravity to work for the patient.
In the past 30 years there has be a reduction in the incidence of LTS particularly in the neonates. Seven studies looking at the years of 1971-1979 found an incidence of neonatal SGS of 0.9% to 8.3%. Nine studies looking at the years of 1980 to1989 reported an incidence of neonatal SGS of 0.0% to 4.2% and 4 studies describe patients cared for between 1990-1999 and reported an incidence of neonatal SGS of 0.0% to 0.63%. Overall the incidence today has been estimate to be 0% to 2% for intubated neonates (Walner, 2001). This compares to incidence estimates in the 1960s of upwards of 22%. This reduction has most likely resulted from better education of physicians and nurses and an increasing awareness of the factors contributing to the development of SGS. Routine use of artificial surfactant and systemic steroids in premature infants as well as the increased use of CPAP when possible has also contributed to the decline in the incidence of SGS.
Vocal Cord Paralysis and Vocal Cord Medialization
Anatomy
The cartilages of the larynx consist of the thyroid cartilage, the epiglottis, the cricoid cartilage, and the arytenoid cartilages. The corniculate and cuneiform cartilages stiffen the aryepiglottic folds. The arytenoid cartilages articulate with the cricoid by means of a true synovial joint. This joint allows two movements of the arytenoid cartilages – rotation and lateral gliding.
There are three groups of intrinsic laryngeal musculature – the abductors, adductors, and tensors. The only abductor of the larynx is the posterior cricoarytenoid muscle and it is innervated by the recurrent laryngeal nerve. The adductors are composed of the lateral cricoarytenoid muscle, interarytenoid muscle, oblique arytenoid muscles, and thyroarytenoid muscles. Innervation of the adductors is again supplied by the recurrent laryngeal nerve. The tensors are composed of mainly the cricothyroid muscle, which is innervated by the external branch of the superior laryngeal nerve, and to a lesser extent by the thyroarytenoid muscles.
The true vocal folds have an epithelial lining that is composed of respiratory epithelium (pseudostratified squamous) on the superior and inferior aspects of the fold and nonkeratinizing squamous epithelium on the medial contact surface. The subepithelial tissues are composed of a three-layered lamina propria based on the amount of elastin and collagen fibers. The superficial layer is composed of mostly amorphous ground substance and contains a scant amount of elastin with few fibroblasts – this layer is termed Reinke’s space. The intermediate layer has an increased elastin content. The deep layer has less elastin but a greater amount of collagen fibers. The intermediate and deep layers have a higher concentration of collagen fibers and are termed the vocal ligament. Deep to the lamina propria is the thyroarytenoid (or vocalis) muscle. Reinke’s space and the epithelial covering are responsible for the vocal fold vibration.
The Vagus:
Understanding the anatomy of the vagus nerve is important because branches of the
vagus nerve are responsible for innervation of the larynx. The vagus nerve has three nuclei located within the medulla:
- The nucleus ambiguus
- The dorsal nucleus
- The nucleus of the tract of solitarius
The nucleus ambiguus is the motor nucleus of the vagus nerve. The efferent fibers of the dorsal (parasympathetic) nucleus innervate the invuluntary muscles of the bronchi, esophagus, heart, stomach, small intestine, and part of the large intestine. The efferent fibers of the nucleus of the tract of solitarius carry sensory fibers from the pharynx, larynx, and esophagus.
Vagus means “wanderer” which is appropriate for the path this nerve takes after emerging from the jugular foramen. It has two ganglia, the smaller superior ganglion and the larger inferior, or nodose, ganglion. The vagus sends small meningeal branches to the dura of the posterior fossa and an auricular branch, which innervates part of the external auditory canal, the tympanic membrane, and skin behind the ear. In the neck, the vagus runs behind the jugular vein and carotid artery to send pharyngeal branches to the muscles of the pharynx and most of the muscles of the soft palate. The superior laryngeal nerve separates from the main trunk of the vagus just outside the jugular foramen. It passes anteromedially on the thyrohyoid membrane where it is joined by the superior thyroid artery and vein (see vasculature). At approximately this level, the external laryngeal nerve leaves the main trunk. The main internal laryngeal nerve enters the thyrohyoid membrane through a hiatus. It then divides into three set of branches (ascending, transverse and descending), which communicate with the recurrent laryngeal nerve posterior to the cricoid cartilage; this is referred to as the ansa galeni. The internal superior laryngeal nerve penetrates the thyrohyoid membrane to supply sensation to the larynx above the glottis. The external superior laryngeal nerve runs over the inferior constrictor muscle to innervate the one muscle of the larynx not innervated by the recurrent laryngeal nerve, the cricothyroid muscle.
The right vagus nerve passes anterior to the subclavian artery and gives off the right recurrent laryngeal nerve. This loops around the subclavian and ascends in the tracheo-esophageal groove. It tends to run with the inferior thyroid artery for part of its course before it enters the larynx just behind the cricothyroid joint. It may branch prior to this with sensory fibers supplying sensation to the glottis and subglottis. The left vagus does not give off its recurrent laryngeal nerve until it is in the thorax, where the left recurrent laryngeal nerve wraps around the aorta just posterior to the ligamentum arteriosum. It then ascends back toward the larynx in the TE groove. The vagus then continues on into the thorax and abdomen contributing fibers to the heart, lung, esophagus, stomach, and intestines as far as the descending colon.
Normal function/movement/physiology
The larynx has a variety of functions. It acts as a sphincter to close the airway during swallowing, preventing aspiration of food and liquids. This is phylogenetically the oldest and perhaps most important function of the larynx. Its function is also essential for respiration. Since the larynx is the gateway to the airway, laryngeal disease may result in obstruction of the airway. It functions during communication of both intellectual and emotional expression. Thus, voice deterioration is only one symptom of laryngeal dysfunction. It also stabilizes the thorax by preventing exhalation, this helps stabilize the arms during lifting. During coughing, lifting, and straining it compresses the abdominal cavity. Aspiration on swallowing, ineffective cough, and breathy voice are symptoms caused by the loss of sphincteric function, and can occur in addition to hoarseness in patients with true vocal fold paralysis.
Phonation is defined as the physical act of sound production by means of passive vocal fold interaction with the exhaled airstream. Basically, this sound production arises from a passive movement of the true vocal cords (TVC)s modified in terms of pitch, quality, and volume by complicated interaction of thoracic and abdominal muscles, intrinsic and extrinsic muscles of larynx, and the shaping and resonance of the upper airway and nasal passages. Contraction of the expiratory muscles produces a rise in subglottic air pressure causing rapid escape of air between the nearly apposed TVCs. Bernoulli’s effect and the elasticity of the cords causes medial displacement of the medial edges of cords and airflow is stopped. A rapid rise again in subglottic pressure causes the cords to part and the cycle is repeated. It is the escape of small puffs of air that produces the vibratory phenomenon interpreted as sound.
During phonation the lower margins of the true vocal folds separate first with formation of a volume of subglottic air. As the upper margins of the vocal folds separate a burst of air is released – the glottal puff. The lower fold then returns to midline, followed by the upper margin. This delay between closure of the lower and upper margins of the fold is termed the phase delay. The mucosal wave consists of both a horizontal movement of the folds and a vertical undulation.
The body-cover theory helps explain this mucosal wave. It states that there are two layers of the vocal folds with different structural properties. The cover is composed of stratified squamous epithelium and the superficial layer of the lamina propria (Reinke’s space). The body of the fold is composed of the intermediate and deep layers of the lamina propria (which is more fibrous than the superficial layer – the “vocal ligament”) and the thyroarytenoid (vocalis) muscle. The cover is pliable, elastic, and nonmuscular, whereas the body is more stiff and has active contractile properties that allows adjustment of stiffness and concentration of the mass. The mucosal wave occurs primarily in this loose cover of the fold. Changes in stiffness or tension in the fold alters the mucosal wave. As the stiffness in the fold increases – as by contraction of the cricothyroid muscle – the velocity of the wave increases and the pitch rises. Mucosal wave velocity also increases with greater airflow and greater subglottal pressure.
The pitch of voice is related to the fundamental frequency of vocal fold vibration (measured in hertz). The fundamental frequency of vocal fold vibration correlates with changes in vocal fold tension and subglottic pressure. Contraction of the cricothyroid muscles, which correlates positively with vocal fold tension, is the main predictor of fundamental frequency, especially at high frequency. Contraction of the thyroarytenoid may change the tension of the vocal fold cover and body and affect the fundamental frequency also. Three physical properties of the vocal folds determine frequency of vibration – mass, stiffness, and viscosity
Mass – the fundamental frequency of vocal fold vibration is inversely proportional to its mass. Decreasing the mass – thinning of the fold by longitudinal stretching (contraction of the cricothyroid muscle with elongation of the vocal folds) – increases the frequency of vibration. Increasing the mass – contraction of the thyroarytenoid muscle with increased concentration of the fold – will decrease the fundamental frequency.
Stiffness – vocal fold tension is an important variable in the control of fundamental frequency at the mechanical level. Vocal fold tension is affected by the contractile forces of the vocal fold musculature and the tissue characteristics of the vocal fold body, cover, and the connecting fiber structure of the vocal folds.
Viscosity – Viscosity is inversely related to ease with which the tissue layers slip over one another in response to a shear force. Increased viscosity of the vocal folds would require greater subglottal pressure to maintain the same vibratory characteristics. Therefore, hydration of the vocal folds has effect on the voice quality and ease of voice production.
PATHOLOGY AFFECTING VOICE:
Unilateral Vocal Cord Paralysis: When one of the vocal cords is paralyzed, the cords are not able to meet in the midline to initiate the glottic attack. This prevents development of the subglottic pressure needed to initiate speech.. Also with the cords at such a distance, the mucosal wave cannot be adequately maintained. Hoarseness and breathiness are the most common complaints but vocal abnormalities may also include easy fatigability and voice or pitch change. It is important not to assume that the immobile cords are necessarily paralyzed. Arytenoid fixation can lead to an immobile cord and direct palpation of the arytenoid cartilage and/or laryngeal EMG can rule out this possibility. Potential return of function of an immobile cord can be determined if the underlying cause is known and with the aid of LEMG. This contributes significantly to the choice of surgical procedure to correct the problem. It is also important to remember that the larynx has a number of functions in the human and dysphonia may not be the primary compliant. Patients may be suffering from dysphagia, coughing, or choking episodes, or stridor.
There are a number of different causes of unilateral vocal cord paralysis. Any entity affecting the vagus nerve along its course may result in decrease in function. The most common cause is non-laryngeal cancer which includes neoplasms of the head, neck, chest, and skull base. Neuritis associated with upper respiratory infection, syphilis, or other infectious sources may cause nerve dysfunction. Neurologic conditions such as CVA, multiple sclerosis and myasthenia gravis may also effect vocal cord functioning. General medical conditions such as diabetes mellitus may cause an isolated neuropathy giving rise to vocal paralysis. Lesions of the vagal nerve occurring higher in the brain and may present with multiple cranial nerve abnormalities.
Vocal Fold Bowing: The inability of the folds to approximate at the midline decreases the ability to produce proper speech. Though it may be a normal change in the aging patient, it is also seen with muscular atrophy secondary to nerve sectioning or central neurologic conditions. With aging, changes in the lamina propria include a loss of elastic fibers, atrophy of submucous glands, increased fibrosis, and muscle atrophy. These changes result in an increased glottic gap and a number of perceptual changes. Geriatric patients may present with hoarseness, low pitch, imprecise articulation, or breathiness.
PATIENT EVALUATION AND SELECTION:
-History:
GENERAL: As always, obtaining a pertinent history is of utmost importance. One should determine the onset, duration, and severity of the dysphonia. As previously mentioned, the larynx is also crucial in protecting the lower respiratory tract and is a conduit of the upper respiratory tract. Therefore the patient may present with coughing and choking episodes, aspiration, stridor, dyspnea, dysphagia, or odynophagia (2). Intubation history and previous head and neck trauma are crucial pieces of information. It is important to know if the patient has had any previous laryngeal surgery or other head and neck surgery.
VOCAL: A specific vocal history is also important. Many patients who present with vocal complaints have a disease entity that does not warrant surgical treatment. Aside from onset, duration, variability, and past vocal problems, history should include pertinent medical questions such as presence of seasonal allergies, history of reflux disease, life stress, diabetes, and medications. Many patients who present for an initial evaluation of voice complaints are unfamiliar with questions of vocal use and hygiene. It is important for the physician to explain these concepts to the patient during the questioning to facilitate accurate responses and educate the patient. Questions should include voice demands at home and at work, recreational singing, and episodes of abuse i.e. sporting events. Smoking, water intake, caffeine intake, and environmental irritants are important questions about vocal hygiene.
-Physical:
It is important to do an entire exam with emphasis on palpation of the neck to assess for any neck mass or goiter and cranial nerve testing. An indirect laryngeal exam, as well as a flexible laryngoscopy or videostrobe should be performed. The patient should phonate a high pitched /ee/ sound. This causes elongation of the vocal folds and causes the larynx to move superiorly. These movements aid in obtaining a complete view of the larynx. In addition to assessing vocal fold position and mobility, it is crucial to rule out carcinoma of the larynx in a patient presenting with hoarseness. A direct laryngoscopy with palpation of the arytenoids to ensure joint fixation is absent should be done prior to any surgical procedure.
The manual compression test is an easy non-invasive office procedure to help evaluate a number of voice disorders. The lateral manual compression test is particularly useful in determining whether a patient with a wide glottic gap from unilateral vocal cord paralysis or vocal bowing will benefit from a medialization thyroplasty. To perform the test, the neck should be palpated to find the superior notch and the inferior margin of the thyroid ala. The vocal cords are located along a horizontal line drawn at the midpoint of these two landmarks. The patient is asked to sustain an /a/ phonation and pressure is applied to the lateral aspects of the thyroid cartilage. The concept is to approximate the vocal folds and decrease the glottic gap. A subjective improvement in voice quality is sufficient to state that the patient would benefit from a medialization thyroplasty though acoustic, aerodynamic, and videostroboscopic studies can be done to quantify improvement. The limitations to this test are older patients who have calcification of the thyroid cartilage, patients with obese necks, and patients with scarring of the vocal folds.
Vocal Assessment:
Despite the recent outburst of technology used to measure and quantitatively assess voice, there is no substitute for the trained ear. Taking a history gives ample time for the physician to make a qualitative assessment of the patient’s voice. Qualities such glottic fry, hard glottal attacks, breathiness, diplophonia, pitch breaks, phonation breaks, and tense phonation can be assessed.
Acoustic evaluation is the quantitative measurement of various voice characteristics. Having the patient sustain a single tone, the fundamental frequency (Fo), variations in amplitude (shimmer), and variations in pitch (jitter) can be measured. Fo may be decreased in patients with vocal abuse or poor approximation of the vocal folds. Shimmer alteration is due to decreased stability of the vocal folds. Abnormal jitter correlates with the subjective quality of hoarseness.
Videostrobolaryngoscopy (VSL) should be performed whenever possible. It allows for dynamic assessment of the vocal folds. With this view, the physician is able to differentiate between functional voice problems and those caused by subtle structural abnormalities. Pulses of light allow us to watch various parts of successive cycles to obtain a complete picture of vocal cord activity. The physician is able to evaluate symmetry of movement, aperiodicity, glottic closure configuration, and horizontal excursion amongst other variables. If the cords are functioning symmetrically, they should essentially be mirror images of each other. The lateral excursion and timing of opening/closing should be identical. Aperiodicity is a measure of irregularities in vocal fold movement. If the frequency of the strobe light is equal to the fundamental frequency, no vocal fold movement should be seen. If movement is observed followed by a static period, aperiodicity is present. The glottis may also be assessed for gap, shape, and appropriate closure (11). The shape of the glottis may be characterized as complete, anterior chink, irregular, bowed, posterior chink, hourglass, or incomplete. Horizontal excursion is a measurement of the amplitude of the cords. Measurement both pre and post-operatively can provide objective data for evaluating improvement. An additional benefit is reviewing the results with the patient immediately after performing the examination. Giving the patient a visual image of the problem helps considerably in motivation for behavioral treatment and development of goals for improvement.
Electromyography (EMG), though not routinely performed, is an excellent evaluation of specific muscle functioning. By placing electrodes into laryngeal muscles (thyroarytenoid, cricothyroid), EMGs help elucidate whether there is any re-innervation of muscles which are thought to be paralyzed. It can also help to differentiate paralysis from arytenoid joint fixation. EMGs are also used to identify excessive muscle activity prior to the use of BOTOX for spasmodic dysphonia.
Diagnostic Tests:
If indirect or stroboscopic exam demonstrates a unilateral vocal cord paralysis with no known etiology, a specific battery of tests should be considered. A CT scan from skull base to the mediastinum should be done to evaluate the entire length of the vagus and recurrent laryngeal nerves. If the patient is a child, pregnant, or suspected to have a generalized neurologic problem, an MRI is advised instead. A barium swallow may be done to evaluate swallowing mechanism and associated dysphagia. Radioactive thyroid uptake scan or ultrasound may be done to evaluate for the presence of a nodule or tumor. Chest x-ray is performed to rule out the presence of a bronchogenic carcinoma, mediastinal adenopathy/mass, or less likely, the presence of an enlarged heart compressing the recurrent laryngeal nerve, particularly on the left side. A FTA-Abs test should be done to rule out syphilis as a cause of vocal cord paralysis.
TREATMENT OPTIONS:
The most important aspect of rehabilitating voice is defining the patient’s goals.
–VOICE THERAPY :
Assessment of patients by a speech pathologist allows for maximal medical treatment to be implemented before consideration is given to surgical treatment. Some patients develop hyperfunctional compensatory mechanisms which lead to the common complaints of voice strain, neck discomfort, and fatigue (16). Speech pathologists can help eliminate these habits and educate the patient on proper compensation techniques. Relaxation exercises, aerobic conditioning, voice exercises and other methods are all practiced by the patient to improve voice quality. Once vocal therapy has been maximized and further voice improvement is desired, surgical options may be considered. Utilizing voice therapy in treatment of unilateral vocal cord paralysis is crucial to ensuring the greatest improvement in voice.
–CORD INJECTION:
Teflon
Indications: Teflon injections are most commonly used for unilateral vocal fold paralysis with no hope for return of function in terminal patients. To ensure that function will not return, a waiting period of one year is usually observed prior to performing the procedure.
Contraindications: Experience has shown that Teflon injections are particularly poor when the voice complaint is secondary to vocal cord atrophy, or vocal fold bowing.
Procedure: There are a number of different approaches to injecting the vocal folds. When performing the percutaneous injection, no sedation is required and local anesthetic is used. Fiberoptic laryngoscopy is used concurrently to assure proper placement and adequacy of the injection. The lateral percutaneous approach requires the surgeon to pierce the thyroid cartilage at the level of the vocal fold. An anterior approach may be used by placing the needle through the cricothyroid membrane and angling the needle superiolaterally under direct visualization. The Teflon should be placed lateral to the vocalis muscle with great care not to disturb the endolaryngeal mucosa. The first injection should be placed anterolateral to the vocal process of the arytenoid. Teflon is injected until appropriate medialization is seen with fiberoptic laryngoscopy. Another bolus of Teflon is placed anterior to the junction of the middle and anterior one third of
the cord. A transoral injection may be done under local anesthesia using indirect mirror laryngoscopy. It is extremely important to bevel the needle away from the mucosal edge to avoid an intramucosal injection. If the procedure cannot be adequately performed under local anesthesia, it may be done during a direct laryngoscopy under general anesthesia with jet ventilation. It is important not to place excessive pressure on the anterior commissure to avoid distorting the vocal cords. The needle is placed lateral to the vocal fold, 2mm deep, at the level of the vocal process. The patient is asked to phonate and further injections depend upon voice quality. It is important to asses voice quality during the procedure. If too much Teflon is injected, the results may be disastrous. If overinjection does occur, it is imperative to incise the mucosa over the site of injection and suction out the excess.
Advantages: The procedure is inexpensive and produces immediate results. It can also be done under local anesthesia and usually results in satisfactory voice. It is important to note that these advantages, once exclusive to Teflon injection, can be provided by other surgical procedures.
Limitations: The irreversibility of the procedure is a major concern. Teflon may only be placed in a vocal cord which has no potential for return of function. As stated above, this requires one year of waiting after initial presentation to ensure complete paralysis. The only exceptions to this is the terminally ill patient with aphonia or aspiration. If vocal fold function does return after placement of Teflon, voice quality will be poor with increased likelihood of displacement, extrusion, and granuloma formation. Teflon injection into a mobile cord will cause hardening of the cord and disruption of the normal mucosal wave. Attempts to remove a Teflon implant usually result in destruction of the vocal fold. The inability to use Teflon in cases with absent soft tissue is another criticism. This automatically eliminates its use in patients with atrophy and bowing of the vocal folds, status post cordectomy, and status post blunt laryngeal trauma (9). The injection of Teflon is not sufficient to medialize the cord and enhance vocal function. Patients suffering from a central neurologic problem also receive no benefit from Teflon injection. Central lesions typically disrupt superior laryngeal and pharyngeal function and therefore a procedure which narrows the glottic gap may not be sufficient to prevent aspiration. Migration of the implant and extrusion through the vocal membrane are other possible complications. Granuloma formation is the most feared complication. It can result in poor voice quality and eventually airway compromise. Because of this, Teflon is now limited by most.
Collagen injections are derived from bovine collagen which is modified to minimize host immune response. Collagen implants are assimilated into the surrounding tissues by an invasion of fibroblasts and deposition of new host collagen. Histologically, the collagen is similar to the deep layer of the lamina propria. Therefore, the collagen is placed within this layer of the vocal fold. Though there is some resorption of the collagen, this is offset by the deposition of host collagen thereby providing long term voice improvement. Resorption of the collagen may be precipitated by an upper respiratory infection. There have been reports of hypersensitivity reactions with rare cases of airway compromise with the use of Bovine collagen, Zyderm. Some authors still advocate the use of dermal skin tests to test for possible allergic reaction to the injections. In a series by Ford and Bless, 2 of 80 patients had a positive skin test which is consistent with the reported incidence of 3%. Recently, an increased used of Cymetra, a form of collagen composed of micronized homologous alloderm, has decreased the incidence of allergic reactions and lengthened the period of benefit.
Autologous Fat
In 1987, Brandenburg et al. reported the first use of autologous fat injection for glottic insufficiency. Since then, fat injection for a variety of etiologies has become very popular.
Indications: Fat injections have been used successfully in patients with vocal cord paralysis, vocal fold scarring, vocal fold atrophy, and intubation defect.
Contraindications: There are no definitive contraindications to fat injection
Technique: (as described by Hsiung et al. (12)). Under general anesthesia, fat is harvested from the lower abdominal pannus. The fat is cut into 1mm pieces separating it from connective tissue. The fat is then rinsed with lactated ringers followed by a methylprednisolone solution. It is then loaded into a syringe. The actual location of fat placement is dictated by the underlying pathology. For those patients with vocal cord atrophy and paralysis, the anterio- and posteriolateral areas of the middle third of the cord are injected. Injection is continued until a 50% overcorrection and convex bowing of the affected cord is seen.
Outcome: Since its first use in 1987, fat injections have gained popularity. Autologous fat is well tolerated in the vocal cord and repeated injections can be done if necessary. Unlike Teflon where overinjection can be disastrous, placing too much fat in the vocal fold does not cause significant post-operative complications. Overinjection is recommended because a certain percentage of fat will atrophy over time. Postoperative analysis reveals an improvement in glottic closure and mucosal wave production. Though there is an improvement in the breathy quality in those patients with glottic insufficiency, vocal roughness persisted after the procedure. Anterior defects corrected with fat injection have a better postoperative outcome than posterior defects.
Hsiung et al. (12) divided failure into two categories, early and late. With early failure, it was believed that it was due to 1) a large glottal gap or 2) a posterior defect not corrected with fat injection. Late failure was attributed to absorption of the fat supported by an initial improvement in voice quality.
There are still a few concerns and questions about fat injection. Knowing that there will be some reabsorption of the fat, the cord needs to be overinjected. This leads to the question of exactly how much fat results in an optimal change in voice. It is also not known whether improved vocal function is due to the amount of fat injected or softening of the vocal cords. Another uncertainty is the rate of fat absorption by the vocal tissue. If initially effective, the benefits of fat injection may last anywhere from three months to several years. Some studies have shown that despite absorption of the fat, lipocytes and fibrous connective tissue retain the contour of the vocal cord and provide long term benefit. The exact method of harvesting and preparation of the fat and its relation to absorption is still unknown. Effort should be made to minimize that amount of trauma to the fat during extraction.
Synthetic Injectables:
Calcium Hydroxyapatite (Radiance FN; BioForm) is an injectable material made of small spherules of CaHydroxyapatite. No granuloma formation occurs with this agent. Long term efficacy is currently under study.
Polydimethylsiloxane gel (Bioplastique; Bioplasty) is widely used in Europe for vocal fold medialization, but is not approved for use in the U.S. Sustained phonatory improvement up to 7 years has been shown in some European studies.
–TYPE I THRYOPLASTY
Indications: A Type I thyroplasty was repopularized by Isshiki in 1974. The indications for a Type I thyroplasty are unilateral or bilateral vocal fold paralysis or paresis, vocal fold bowing, and incomplete glottic closure with aspirations.
Contraindications: There are two contraindications for performing a Type I thyroplasty. The first is in patients with a previous hemilaryngectomy. Without the support of the thyroid cartilage, the silastic implant is ineffective in medializing the scarred side. Vocal fold injection is indicated in this case. The second contraindication is previous laryngeal irradiation due to extensive scarring.
Technique: There are many variations in this procedure championed by several authors. Described below, is the technique performed by Netterville et al (6). A horizontal incision is made over the midportion of the thyroid cartilage and the cartilage exposed. A window is created in the thyroid ala approximately 8mm posterior to the anterior commissure and 3mm superior to the inferior border of the cartilage. This provides a sufficient strut inferiorly to support the implant. After the window is made, the cartilage is removed. Incisions are made in at the inferior, posterior and superior aspects of the inner perichondrium thereby creating a flap. The perichondrium is elevated from the medial aspect of the thyroid ala. While viewing the cords via fiberoptic laryngoscopy, a depth gauge is used to medialize the cords in the anterior, middle, and posterior aspects of the window and the measurements are recorded. These measurements are also taken at the superior and inferior aspects of the window to find the relation between the true and false vocal cords. Using measurements from the various areas of the windows, an implant can be fashioned from a silastic block. The point of maximal medialization is at the level of the vocal process. Very minimal medialization is designed at the anterior commissure to prevent a strained voice. The inferior aspect of the implant is placed in the window and rotated into place. The patient is asked to phonate and voice is assessed. If medialization is not optimal, the implant can be removed and modified. The time of intralaryngeal elevation and implant placement should be minimized to prevent vocal interference by intraoperative edema.
-Variations/Controversies:
Removal of the cartilage window: Some authors feel that the cartilage, if left in place can migrate superiorly and medialize the false vocal cord or ventricle. If the cartilage migrates inferiorly, it may cause overmedialization of the cord resulting in a persistently strained voice quality.
Inner perichondrium: Some authors prefer to leave the inner perichondrium intact stating that it decreases the incidence of graft extrusion. Netterville states that the reason for increased implant extrusion is injury to the ventricle. This occurs more frequently if a paramedian incision is used near the anterior commissure where the ventricle is located very close to the inner perichondrium. He argues that incising the inner perichondrium does not increase implant extrusion secondary to the development of a fibrous capsule around the implant.
Implant material: Though some authors feel that a carved implant allows for precise results, Montgomery et al. (10) reports certain benefits to a pre-made implant. The inner aspect, which medializes the cord, is made of a softer plastic closer to the consistency of the surrounding tissue. The outer half is made of a harder plastic which locks into the thyroid cartilage. This prevents displacement of the cords and eases revision. Hydroxylapatite is a pre-made implant which has minimal tissue reactivity and good biocompatibility with the surrounding tissue. Gore-tex (ePTFE) is another material reported to be of benefit in medializing a paralyzed vocal cord. This material has excellent biocompatibility and can be used to medialize the cord in an incremental fashion. This technique does not require extreme precision in creating the thyroid window or shaping the implant.
Benefits: Type I thyroplasty has had excellent results in voice improvement. The procedure helps to re-establish the mucosal wave in the paralyzed vocal fold. By approximating the vocal membranes, normal anatomic position is re-established and the cords are able to produce sound. The return of an intact mucosal wave is a large reason that this procedure is so effective in improving voice. This improvement is illustrated by an increased Fo and maximum phonation time. Other objective variables such as glottic closure and cord symmetry are also improved. The improvement in aspiration symptoms is even more consistent than the improvement in voice quality. Additional benefits include the ability to monitor vocal improvement during the procedure if performed under local anesthesia. Using a nasopharyngoscope, the surgeon can ensure the implant is at the level of the true vocal cords and not medializing the false cords or the ventricle. It is both adjustable and potentially reversible. The reversibility of the procedure allows its use in a patient with potential return of vocal cord function. The implant can also be revised if the vocal cord continues to atrophy over time. When performing a Type I thyroplasty, it is important to council the patient on the expected voice changes post-operatively. Though initially strong in the operating room, perioperative edema will cause the patient to be hoarse for the first ten days after the procedure. Some have noted an additional period of voice difficulty occurs 4 to 6 weeks after surgery. This eventually improves and the patient’s voice may continue to improve for the next year. Primary medialization thyroplasty occurs at the time of extirpative surgery with known
sacrifice of the recurrent laryngeal nerve in the neck. This procedure is done under general anesthesia and therefore negates the benefit of intraoperative voice evaluation. It is performed primarily in hope to eliminate the need for a tracheotomy and decrease the postoperative rehabilitation time (swallowing and speech) of patients with loss of multiple cranial nerves.
Complications of a Type I thyroplasty include persistent dysphonia, airway obstruction, implant migration, extrusion, hematoma, and infection. Poor voice quality post-operatively may be due to inadequate medialization or over-medialization of the cords. Appropriate voice assessment can only take place 4 to 6 weeks after the operation when all edema has resolved. Despite various techniques to prevent migration, occasionally the implant may move superiorly and medialize the false cord and ventricle. This calls for removal of the implant and replacement with a larger prosthesis. Extrusion into the airway is a serious complication. Though it does not occur frequently, suspicion should warrant a fiberoptic laryngoscopy and subsequent endoscopic extraction if found. Extrusion laterally can be avoided by securing the prosthesis firmly in the thyroid cartilage. In general, complications can be reduced by careful handling of the tissues, limited operative time, and meticulous hemostasis. (2). Type I thyroplasty may not be sufficient to close a large posterior gap. It may difficult to know pre-operatively whether posterior approximation will be needed. One method proposed by Omori et al.(5) is to obtain videostroboscopic measurements prior to surgery. They assessed the posterior glottic gap as a percentage of the membranous vocal fold length. They found that is the posterior glottic gap was larger than 10% of the membranous vocal fold length, the post-operative outcome was worse and a posterior closure procedure may be warranted. If it is determined that the posterior gap is too large either pre or intra-operatively, the surgeon has the option of either creating an implant with a large posterior component or performing an arytenoid adduction (discussed later). Implants that were originally fashioned to medialize the posterior cord did so by pressing on the vocal process of the arytenoid cartilage. It has since been shown that it is more effective to fashion the implant to apply pressure to the muscular process of the arytenoid. Simply stated, the implant should have a large posterior flange, approximately 5mm in thickness to fit between the muscular process and the thyroid ala. The major advantage of this procedure is, unlike arytenoid adduction, that it does not hinder mobility of the vocal folds.
–ARYTENOID ADDUCTION:
There are two major indications for an arytenoid adduction. The first reason is to close a posterior glottic gap. Given that the cricoid overlaps the thyroid posteriorly, a posterior window is not effective in medializing the posterior vocal cord. The traditional Type I thyroplasty has been shown to be ineffective in medializing the posterior cord. A simple way to assess if an arytenoid adduction is necessary is to see if the vocal processes of the arytenoid cartilages touch in the midline when the patient phonates. The second reason is if the vocal folds are not at the same caudal-rostral level. The vocal process of the arytenoid cartilage moves inferior with adduction and superior with abduction. This is due to the cylindrical shape of the cricoarytenoid joint. Some surgeons advocate an intra-operative assessment of the vocal cord medialization. If
after the silastic implant has been placed, there is a persistent posterior gap, an arytenoid adduction is performed.
The procedure is described as it is performed by Isshiki. Using a horizontal neck incision at the level of the vocal cords, the posterior border of the thyroid cartilage is exposed by transecting the strap muscles and detaching the inferior constrictor from the thyroid. It is important to identify the recurrent laryngeal nerve in this area to avoid any damage. The cricothyroid joint is then opened to allow access to the muscular process of the arytenoid cartilage. The piriform sinus mucosa is then elevated with great care to violating the piriform recess. Cricoarytenoid joint is then opened allow exposure of the muscular process. The posterior cricoarytenoid muscle is identified and ligated from the muscular process. Two 3-0 nylon sutures are placed around the muscular process and the surrounding soft tissue. The sutures are then pulled anteriorly through the thyroid ala. The patient is asked to phonate and the appropriate force is determined to provide optimum voice results.
The only significant variation is whether or not to open the thyroarytenoid joint. Some authors believe that opening the joint results in prolapse of the arytenoid cartilage into the laryngeal lumen with overadduction of the posterior commissure.
Arytenoid adduction can be used in conjunction with medialization thyroplasty and re-innervation surgery. Currently, no other procedure corrects for a discrepancy in vocal cord level and few other procedures effectively address a wide posterior chink.
–REINNERVATION SURGERY WITH ANSA CERVICALIS:
Indications: In the past few decades, there has been a surge of interest in reinnervation surgery as a therapy for unilateral vocal cord paralysis. Given that the arytenoid cartilage is mobile and the ansa cervicalis has not been disrupted, reinnervation with a nerve-muscle pedicle or recurrent laryngeal nerve – ansa cervicalis nastomosis should be considered.
Contraindications: If there is any fixation of the arytenoid cartilages, a nerve anastomosis should not be used. This procedure cannot be performed on a patient who has had disruption of the ansa cervicalis, either by surgery, trauma, or neurological process.
Neuromuscular pedicle reinnervation: An incision is made in the lower half of the thyroid ala extending to the sternocleidomastoid muscle. The ansa cervicalis is identified overlying the jugular vein and is traced to its insertion to the anterior belly of the omohyoid muscle. Two stay sutures are placed 2-3mm proximal and distal from the insertion site. A window is made is similar to that used for a Type I thyroplasty. The inner perichondrium is opened and the thyroarytenoid is incised superficially. Using the stay sutures, the muscle pedicle is sown in place. It is crucial to avoid excessive tension on the pedicle.
Ansa Cervicalis – Recurrent Laryngeal Anastomosis:The ansa cervicalis is exposed overlying the great vessels or within the carotid sheath. The ansa is traced to either the omohyoid or sternothyroid. The nerve is sectioned at its insertion to the muscle and transposed to the tracheoesophageal groove. The recurrent laryngeal nerve is identified by retracting the superior thyroid neurovascular bundle and followed to its insertion into the larynx. The nerve is ligated 7 –10mm from its insertion in the larynx to ensure a tension free anastomosis. The nerves are anastomosed with a neurorrhaphy (epineural repair) with 10-0 suture under magnification.
Outcomes: Re-innervation surgery has recently gained popularity in those patients with unilateral vocal cord paralysis. Though cord injections, medialization thyroplasties, and arytenoid adduction are sufficient to medialize the cord and close the glottic gap, none of these procedures address vocal fold tone, another important component of speech production. Reinnervation surgery provides tone to the thyroarytenoid muscle and gives tension to the vocal fold. Another reason cited to perform reinnervation is to prevent vocal fold atrophy. If a medialization procedure is performed, it may need to be revised 2 to 3 years later because cord atrophy has resulted in an increased glottic gap. Laryngeal reinnervation maintains the bulk of the paralyzed fold. Currently it is not known as to the optimal time to perform reinnervation surgery and which patients it will benefit. It has been proposed that intraoperative EMG can distinguish those patients with no spontaneous reinnervation from those with inappropriate reinnervation (synkinesis). Those patients with no spontaneous reinnervation would be more likely to benefit from operative reinnervation.
A universal criticism of reinnervation is the 4 to 6 month period required for the procedure to be effective. Many authors advocate the concurrent use of a medialization procedure, either Gelfoam injection or thyroplasty. Tucker has described removing the posterior inferior aspect of the implant in order to allow room for the muscle-pedicle implant to be placed.
When comparing the two methods of reinnervation, it is currently unclear which procedure produces the best results. Preliminary work by Hall et al. indicates that the muscle pedicle allows for more rapid innervation and stronger contractile force. Current research is directed toward understanding the role of cell adhesion markers in the role of nerve regrowth. This research will likely have a significant impact on the methods of reinnervation surgery.
Recently a modification has been proposed to the recurrent laryngeal nerve – ansa recurrent laryngeal anastomosis procedure. Paniello (16) has proposed a recurrent laryngeal – hypoglossal nerve anastomosis. The theoretical advantage is that these are the only two nerves involved in swallowing and phonation. Other advantages are an abundance of axons in the hypoglossal nerve, use in patients in which ansa is unavailable, and low donor site morbidity. Initial work with the procedure suggests that it results in a stronger reinnervation and sphincter-like action on swallowing. Though there is denervation of the ipsilateral tongue, no increase in aspiration has been shown
Bilateral Vocal Cord Paralysis:
In contrast to unilateral vocal cord paralysis, voice quality is not the primary concern in patients with bilateral vocal cord paralysis. The significant problem is airway compromise. This can range from unnoticeable to, more commonly, dyspnea and stridor. The patient’s voice quality is usually only mildly affected (if just the recurrent laryngeal nerves are involved) because the paralyzed cords tend to assume the natural position for phonation.
There are three basic ways that bilateral vocal cord paralysis is managed:
- Tracheotomy
- Vocal cord lateralization
- Reinnervation
Tracheotomy:
Tracheotomy has the advantages of providing immediate relief of airway restriction. It can be performed under local anesthesia, and has relatively little reduction in voice quality. Disadvantages include the creation of a stoma that has both cosmetic and long-term care problems, and the need to occlude the tube or wear a speaking valve to phonate. This may be the best option for many patients because it controls the airway while preserving voice quality. In many patients, the tracheotomy can be occluded the majority of the time. In times of exertion, while sleeping, or when the patient has a cold or other respiratory condition, the tracheotomy can simply be unplugged.
Vocal Cord Lateralization:
This involves several techniques that surgically widen the glottic opening. While this improves the airway, the patient’s voice quality suffers. The three most commonly utilized techniques are arytenoidectomy, arytenoidopexy, and cordectomy/cordotomy.
Arytenoidectomy:
Classic arytenoidectomy involves removal of some or all of the arytenoid cartilage. This procedure can be performed in a variety of ways, from endoscopically by microsurgical or laser technique to an external, lateral neck approach (Woodman). The Woodman procedure involves a lateral neck incision, exposure of the arytenoid cartilage posteriorly with removal of the majority of the cartilage, sparing the vocal process. A suture is then placed into the remnant of vocal process and fixed to the lateral thyroid ala. This technique seems to cause less voice deficit than other approaches.
Arytenoidopexy:
Arytenoidopexy displaces the vocal fold and arytenoid without surgical removal of any tissue. It can be done endoscopically with a suture passed around the vocal process of the arytenoid and secured laterally. This procedure, however, has a relatively high failure rate and is technically difficult.
Cordectomy:
Dennis and Kashima (1989) introduced the posterior partial cordectomy procedure using the carbon dioxide laser. This involves excising a C-shaped wedge from the posterior edge of one vocal cord. If this posterior opening is not adequate after 6-8 weeks, the procedure can be repeated or a small cordectomy can be performed on the other vocal cord. Laser cordotomy removes a smaller posterior portion of the true vocal cord and better preserves voice.
Reinnervation:
Tucker proposed a nerve-muscle transfer to the posterior cricoarytenoid muscle for the treatment of bilateral vocal cord paralysis. The technique is similar to the one used for unilateral vocal cord paralysis. Prerequisites are that the cricothyroid joint not be fixed and that the necessary nerve for the graft not have been affected by the process that caused the paralysis. Tucker reports a high success rate.
Pediatric Congenital Subglottic Stenosis
Anatomy
The subglottis is defined as the area extending from the lower surface of the true vocal cords to the lower surface of the cricoid cartilage. In adults this corresponds to approximately 10 mm inferior to the anterior commissure and 5 mm inferior to the posterior commissure.
The infant larynx differs significantly in size and position when compared to the adult larynx. At birth, the infant larynx is approximately one third the size of the adult larynx, however, the infant larynx is proportionately larger than the adult larynx compared with the remainder of the tracheobronchial system. The vocal process of the arytenoid takes up half the length of the vocal cord in the infant larynx, while it only takes up about ¼ of the length of the vocal cord in the adult. The narrowest portion of the airway in the older child and adult is the glottic aperture, while the narrowest part of the airway in the infant is the subglottis. The subglottis in infants measures approximately 4.5 by 7mm. A diameter of 4.0 mm is considered the lower limit of normal in a full term infant and 3.5 mm in a premature infant. One millimeter of edema circumferentially in the subglottis reduces the cross-sectional area by 60%.
The infant larynx is positioned higher in the neck than the adult larynx. The superior border of the larynx of the infant is located at about the level of the first cervical vertebrae with the cricoid positioned at about the fourth cervical vertebrae. In comparison, the adult cricoid rests at about the level of the sixth cervical vertebrae. The structures of the infant larynx are more pliable and less fibrous making the infant airway more susceptible to narrowing from edema and less easily palpable.
Embryology
The anlagen of the larynx, trachea, bronchi and lungs arise from the ventromedial diverticulum of the foregut called the tracheobronchial groove at day 25. The lining of the larynx and trachea are from endoderm, while the muscles and cartilage are derived from splanchnic mesenchyme. At day 33 the laryngeal primordia appears, and the laryngeal aditus is formed by the growth of three tissue masses, the hypobranchial eminence from arches III and IV (epiglottis), and the paired ventral ends of arch IV (arytenoids), giving it a T-shape. This slit, extends to the first tracheal ring in the fifth and sixth week, and by week seven the cricoid ring is complete, and the cartilaginous hyoid is visible below the epiglottis. By the end of the embryonic period, the larynx and trachea are well formed.
During the fetal period, the structures are refined, and neurological reflexes are developed. In the third month, the thyroid laminae fuse, cartilaginous vocal processes of the arytenoids are seen, and the ventricle and saccule are seen. The fourth month reveals laryngeal tissue with goblet cells, and tracheal tissue with cilia. In the fifth and sixth months, the cuneiform and corniculate cartilages develop, and the epiglottis has fibroelastic cartilage. Throughout the remaining fetal course, breathing becomes a more mature and coordinated event.
Etiology
- Congenital SGS
- Membranous
- Increased fibrous connective tissue
- Hyperplastic submucous glands
- Granulation tissue
- Cartilaginous
- Cricoid cartilage deformity
- Small cricoid
- Elliptical cricoid
- Large anterior lamina
- Large posterior lamina
- Generalized thickening
- Submucous cleft
- Trapped first tracheal ring
- Acquired SGS
- A. Intubation
- B. Laryngeal trauma
- Previous airway surgery
- High tracheotomy
- Cricothyroidotomy
- Pprior surgery for respiratory papillomatosis
- Prior laser surgery for SGS
- Accidental
- Inhalational (thermal or caustic)
- Trauma (blunt or penetrating)
- Autoimmune
- Infection
- Gastroesophageal reflux (GER)
- Inflammatory diseases
- Anti-neutrophil Cytoplasmic Autoantibodies (C-ANCA)
- Sarcoidosis
- Systemic lupus erythematosis
- Neoplasms
- Idiopathic SGS
Congenital Subglottic Stenosis
Subglottic stenosis is the third most common cause of stridor in the neonate behind laryngomalacia and vocal cord paralysis. Involves narrowing of the subglottic lumen in the absence of trauma (intubation). Normal diameter of newborn trachea is 5mm, lumen of 4mm in full-term newborn (3mm in premature infant) represents subglottic stenosis. Proposed mechanism is incomplete recannalization during embryogenesis.
There are two types of congenital subglottic stenosis: membranous and cartilaginous. Membranous is usually circumferential, soft and dilatable, in contrast, the cartilaginous has a more variable appearance. Mild-normal shape with narrowed lumen, or can have abnormal shape of cricoid cartilage with prominent lateral shelves giving an elliptical appearance to the lumen.
Symptoms
Symptoms of upper airway obstruction dominate, with inspiratory stridor, progressing to biphasic as obstruction worsens. Stridor is an important symptom of SGS as well as many other causes of neonatal obstruction. The characteristics of stridor can be a clue as to where the obstruction is occurring. Three distinct zones have been identified, the supraglottic/supralaryngeal zone, the extrathoracic tracheal zone which includes the glottis and subglottis, and the intrathoracic trachea. Supraglottic stridor is high-pitched, and inspiratory. An example of this is laryngomalacia, where the tissues are floppy and are sucked in during inspiration causing narrowing of the airway. Glottic and subglottic stridor is usually biphasic in nature because these structures are rigid, and there is no collapse of tissue during either phase of respiration, there is a fixed obstruction. Intrathoracic stridor has a expiratory noise, this is due to the positive pressure exerted on the bronchial tissues by contraction of the thorax.
Causes of stridor in children according to site of obstruction
- Pharynx
- Congenital
- Lingual thyroid
- Choanal atresia
- Craniofacial anomalies
- Cysts
- Inflammatory
- Abscess
- Allergic polyps
- Neoplasm
- Adenotonsillar hypertrophy
- Foreign body
- Larynx
- Congenital
- Laryngomalacia
- Webs, cysts, laryngocele
- Subglottic stenosis
- Laryngeal cleft
- Inflammatory
- Croup, epiglottitis
- Tb, rare infections
- Neoplasm
- Subglottic hemangioma
- Laryngeal papilloma
- Cystic hygroma
- Malignant (chondrosarcoma, rhabdomyosarcoma)
- Trauma
- Intubation
- Foreign body
- Neck trauma
- Vocal cord paralysis
- laryngospasm
- Tracheobronchial tree
- Congenital
- Vascular anomalies
- Webs, cysts
- Tracheal stenosis
- Neoplasm
- Foreign body
- Trauma
- Intubation
- Tracheotomy
In addition to stridor, may show intercostal or xyphoid retractions and have a barking cough. Mild-moderate SGS is exacerbated by URI, and these children tend to have recurrent croup.
Diagnosis
When these children present, it is important to perform a thorough history and physical exam. Birth injury, or intubation, as well as prematurity are important to note. The timing, onset, and duration of stridor, voice/cry quality, feeding abnormalities or failure to thrive, cyanosis, and possible foreign body aspiration are important to document. Also, recurrent croup or hospitalizations for respiratory illnesses. The physical exam should include a thorough head and neck exam, as well as careful characterization of stridor, and signs of respiratory distress. A flexible laryngoscopic exam should also be performed. At that time, an assessment of laryngomalacia, vocal cord paralysis, laryngopharyngeal refluxx, or other laryngeal pathology can be ellicited.
Differential diagnosis of laryngotracheal stenosis
- Congenital
- Tracheomalacia
- Laryngomalacia
- Vocal cord paralysis
- Laryngeal cleft
- Congenital cysts
- External compression from congenital abnormality or lesion
- Vascular compression
- Innominate artery compression (most common)
- Right-sided aortic arch with persistent ductus arteriosus
- Aberrant left pulmonary atery
- Mass
- Teratoma
- Cystic hygroma
- Hemangioma
- Infectious/inflammatory
- Viral laryngotracheobronchitis (croup)
- Retropharyngeal abscess
- GER
- Tracheitis
- Neoplastic
- Subglottic hemangioma
- Recurrent respiratory papillomatosis
- Traumatic
External compression
Foreign body
The gold standard for diagnosis of any laryngotracheal abnormalities is direct laryngoscopy and tracheobronchoscopy under general anesthesia. This should be performed in the operating room with an experienced anesthesiologist. It is important to delay endoscopy for at least two weeks following an acute episode of croup to minimize the risk of postoperative airway obstruction. The potential need for tracheotomy should be discussed with the patient (adults) or patient’s family (children) prior to endoscopy. A rigid bronchoscope or a rod lens telescope may be used to assess the airway. The important things to document during endoscopy are as follows: (1) the outer diameter of the largest bronchoscope or endotracheal tube that can be passed through the stenotic segment, (2) the location/subsites (glottis, subglottis, trachea) and length of the stenosis, (3) other separate sites of stenosis, (4) other airway anomalies in infants (clefts, webs, cricoarytenoid joint fixation, neoplasms, etc.), and (5) reflux changes. After removing the sizing endotracheal tube or bronchoscope it is important to observe the stenotic segment for edema which may result in the need for tracheostomy
There are two widely excepted staging systems for classifying subglottic stenosis: Myer-Cotton grading system and the McCaffrey system. Other systems have been described as well, however, none are universally applicable or useful. At this time, no staging system exists that allows comparison of patients treated at different institutions.
The Myer-Cotton staging system is useful for mature, firm, circumferential stenosis confined to the subglottis. It describes the stenosis based on the percent relative reduction in cross-sectional area of the subglottis which is determined by differing sized endotracheal tubes. Four grades of stenosis are described with this system: grade I lesions have less than 50% obstruction, grade II lesions have 51% to 70% obstruction, grade III lesions have 71% to 99% obstruction, and grade IV lesions have no detectable lumen or complete stenosis.
The McCaffrey system classifies laryngotracheal stenosis based on the subsites involved and the length of the stenosis. Four stages are described: stage I lesions are confined to the subglottis or trachea and are less than 1cm long, stage II lesions are isolated to the subglottis and are greater then 1 cm long, stage III are subglottic/tracheal lesions not involving the glottis, and stage IV lesions involve the glottis.
Management
The management of congenital SGS ranges from observation with supportive care in times of exacerbations, to complicated surgical reconstructions of the patients airway. Most grade I and II lesions are managed with observation. If a grade II lesion becomes symptomatic, causing a decrease in exercise tolerance or respiratory distress, then endoscopic repair, dilation, or an expansion procedure can be undertaken. KTP and CO2 laser has also been used for lesions that are thin, and circumferential. Failures tend to occur with thick, circumferential cicatricial scarring greater then 1 cm in vertical dimension, and posterior commissure involvement. Complications of laser include chondritis, and perichondritis, as well as re-stenosis. With high grade stenosis, grade III-IV will need an open surgical procedure.
Surgical treatment options for high grade subglottic stenosis:
- Tracheostomy
- Open procedure
- Expansion procedure (one-stage or with stent placement)
- Anterior cricoid split with or without cartilage graft
- Posterior cricoid split with or without cartilage graft
- Anterior and posterior cricoid split with cartilage graft
- Four quadrant LTR
- Segmental resection (cricotracheal resection – CTR)
- Primary CTR
- Salvage CTR
- Extended CTR – CTR with and expansion procedure, arytenoid lateralization, or aytenoidectomy
The goal of surgery is to have patients with adequate airway to allow for normal activity without the need for tracheostomy. Secondary goal to have single-stage procedure, minimal postoperative morbidity, and minimal hospital stay. Most surgeries are performed in spring and summer, when chance of developing RSV is lower.
Anterior Cricoid Split
The anterior cricoid split (ACS) procedure was originally described for a neonate who has had multiple failed extubations instead of performing a tracheostomy (Cotton and Seid, 1980). This procedure is also used for older infants and those who are have already been tracheotomized. Indications were later expanded to patients with congenital subglottic stenosis. The lesion responsive to this procedure is a mild anterior subglottic narrowing with extensive fibrosis but a normal cricoid. ACS may also be used to decompress subglottic cysts. Strict criteria for ACS have been established by Cotton and include: extubation failure on two occasions or more due to laryngeal pathology, weight >1500g, no assisted ventilation for 10 days prior to evaluation, O2 requirements <30%, no CHF for one month prior to evaluation, no acute respiratory tract infection, no antihypertensive medications ten days prior to evaluation. The procedure is performed after direct laryngoscopic and bronchoscopic confirmation of the diagnosis. All other airway pathology must be ruled-out.
A vertical midline incision is made through the cricoid cartilage and first two tracheal rings as well as the lower thyroid cartilage. This allows the cartilages to spring open and allow edematous mucosa to drain, increasing airway size. Prolene stay sutures are placed on either side of the cricoid cartilage and the skin is re-approximated after placement of a drain. The child is then left intubated, sedated and paralyzed in the ICU for 7-14 days. Cotton has guidelines for endotracheal tube sizes for stenting and for duration of stenting based on the infants weight.
Laryngotracheal Expansion Surgery
Laryngotracheal expansion surgery involves scar division with distraction of the edges by interposition of graft material (augmentation) to widen the airway lumen. It is important to avoid removing scar which results in a large surface area of denuded mucosa and leads to restenosis. Cotton recommends augmenting the airway with grafts when the distraction of the laryngotracheal framework must be greater than approximately 3mm. There are several techniques depending on the location and severity of the stenosis.
Laryngotracheoplasty can be performed with a tracheostomy and formal stenting or by using the endotracheal tube as a stent, the latter known as a single-stage LTP (SS-LTP). Gustafson et all performed a retrospective chart review of 200 patients undergoing SS-LTP. There was a 96% successful decannulation rate, with 29% requiring reintubation. Those undergoing both posterior and anterior grafting had more complications than those undergoing either anterior or posterior grafting alone. Younis et al, performed a retrospective chart review of 46 patients undergoing anterior/posterior SS-LTP and had a 83% decannulation rate. Thus, this procedure should be used for either anterior or posterior stenosis, but other procedures should be considered if there is anterior and posterior stenosis.
Several stents have been used in the past, but the aboulker stent (teflon) is the most used. It has the benefit of being rigid, and having a lumen with a sufficient diameter for aggressive pulmonary toilet. The duration of stenting depends on the length of the prior stenosis, and the grade of stenosis. Stenting can last from four weeks to greater than two months. (Cable et al 2004)
Graft material has included rib cartilage, auricular cartilage, thyroid cartilage hyoid bone, and irradiated cartilage. Autogenous cartilage is the material of choice for grafting, most commonly costal or auricular cartilage. Cartilage is better material because it has a lower rate of resorption, is easy to carve, and is viable without a vascular pedicle. They also retain bulk even without functional use. Hyoid bone requires vascular pedicle, can resorb, and is difficult to carve.
Anterior laryngofissure with anterior lumen augmentation
This technique is good for anterior subglottic stenosis or anterior tracheal wall collapse. The lesion should not involve the glottis. Other procedures should be considered if there the cricoid cartilage is deformed or weak. Anterior grafts are made considerably larger and thicker than grafts placed posteriorly. The perichondrium is oriented to the luminal side to allow for epithelialization. The perichondrium is also a good barrier against infection. A large external flange is created to prevent the graft from prolapsing into the airway.
Laryngofissure with division of posterior cricoid lamina
This is indicated for patients with posterior subglottic stenosis, posterior glottic stenosis that extends to the glottis, complete or circumferential stenosis, or if there is significant cricoid deformity. Division of the anterior and posterior cricoid must be carried out for this procedure. If possible, one should avoid a complete laryngofissure to avoid damaging the anterior commissure, however this is often needed for posterior glottic involvement for access. The posterior cricoid cartilage is incised in a manner that is vertically oriented to the cartilage to allow maximal purchase for the graft. The incision is extended superiorly to the interarytenoid area and inferiorly 5 to10 mm into the membranous trachea. The graft is elliptical in shape. It should not be too thick as it can cause swallowing difficulties and can lead to aspiration. The width of the graft is determined by the desired distraction of the cut edges of the incised posterior cricoid cartilage. 0.05 to 1.00 mm of distraction can be obtained for each year of age, up to 1 cm. It is sutured in place with absorbable suture on a small cutting needle. The knots should be buried so that they remain extraluminal to prevent development of granulation tissue. Long-term stenting is usually necessary (3-6 months).
Laryngofissure and division of posterior cricoid lamina with anterior and posterior grafts
This should be used for patients who have SGS similar to those above but with a significant amount of stenosis posteriorly such that grafting is necessary to maintain the adequate separation.
Once the grafts have been sutured into place in any of the above procedures, the decision must be made on whether it should be single or double-staged. Cotton and Walner (1999) recommend a double-staged procedure for patients with severe stenoses, history of reactive airway, or poor pulmonary function. This should also be considered at institutions with inadequate intensive care facilities. Double-stage procedure implies placement of stent above the tracheostomy tube instead of using an endotracheal tube as the stent (single-staged procedure). Once this decision is made, the strap muscles are closed to provide blood supply to the outer surface of the anterior graft.
Segmental resection/Cricotracheal resection (CTR) with thyrotracheal anastomosis
The first CTR was performed by Conley in 1953 in a patient undergoing surgery for chondroma of the cricoid cartilage. It was later popularized by Ogura and powers (1964) as a technique for treatment of traumatic stenosis. In the 1970s it became the treatment of choice in adults with acquired subglottic stenosis from long term intubation. Until recently, surgeons were reluctant to perform this procedure in the pediatric patients because of the risk of anastamotic dehiscence and recurrent laryngeal nerve injury, and disturbing the normal growth of the larynx. The first successful CTR performed in a child was occurred in 1978 (Savary). In 2005, White et al performed a retrospective chart review of 96 patients undergoing CTR. 89 of these patients had grade III/IV stenosis, and they achieved a 94% decannulation rate. They found that vocal fold dysfunction was the only significant risk factor for failure to decannulate after one procedure.
This technique is indicated if there is severe deformity of the cricoid making grafting very likely to fail. Most say that there must be at least 10 mm of normal airway below the glottis, however Cotton states that the resection can be up to the vocal folds but to expect prolonged edema. This technique is technically difficult due to the close proximity of the vocal cords and recurrent laryngeal nerves. Stenosis less than 4 cm can be resected by laryngeal release and cervical tracheal mobilization. Stenting is not required and the trachetomy tube can usually be removed at around 4 weeks.
Complications
These include bleeding, pneumothorax, pneumomediastinum, recurrent laryngeal nerve injury, slipped graft, slipped stent, plugged stent, wound infection, keloid formation, suprastomal/infrastomal collapse, re-stenosis, tracheocutaneous fistula, granulation tissue and death.
Factors contributing to LTR failure (Choi et al 1999)
Preoperative – inadequate assessment of posterior laryngotracheal stenosis
Intraoperative – duration of stent, type of stent, correct assessment with failure to address posterior stenosis, type of graft, and length of stent.
Postoperative – keloid formation, anterior suprastomal collapse, poor follow-up, slipped stent, broken stent, GERD, no discernable factors.
Postoperative Care
Patients should be admitted to intensive care unit, and care must be coordinated with the ICU team, pediatrics, and otolaryngology. Patients require sedation, but the length of sedation will vary on the age of the child, and the procedure performed. In children over 4 years old, there is a better chance of weaning sedation within 48 hours after the procedure. Aggressive and meticulous thracheostomy care and pulmonary toilet needs to be undertaken. Post operative antibiotics, antireflux medications and dexamethasone are also indicated.
Conclusions
Approximately five percent of children undergoing procedures will require a smaller then expected endotracheal tube due to mild subglottic stenosis. Most of these children will never present to the otolaryngologist, but for more severe cases, these children present a challenging problem for the head and neck surgeon. It is imperative to perform detailed history, physical, and characterization of the extent and severity of the stenosis. Rigid endoscopy is essential for preoperative planning for any of the surgical procedures that can be used for correction. Choice of operation is dependent on surgeon comfort, postoperative capabilities, and severity of disease. For high grade stenosis, the single-stage laryngotracheal resection, or the cricotracheal resection are the best options. CTR is also available as a salvage to LTR failures. Remember that the goal of surgery is to allow for an adequate airway for normal activity without the need for tracheostomy