Pharynx & Larynx-2
CONGENITAL LARYNGEAL ANOMALIES
Introduction
Conngenital abnormalities of the larynx present the otolaryngologist with a wide range of problems ranging from mild to severe. Such anomalies may present at the moment of birth with symptoms requiring immediate attention or may be more insidious in presentation. Thus, it is essential that all otolaryngologists treating children be aware of and have a firm understanding of these conditions which arise from aberrations in embryologic development.
Normal Anatomy
The larynx is a complex, evolutionary structure that permits the trachea to be joined to the pharynx as a common aerodigestive pathway. The larynx is essential in several important functions: 1) ventilation of the lungs, 2) protection of the lungs during swallowing, 3) clearance of secretions by cough, and 4) production of sound. The survival of the infant is predicated on the structural and neurologic integrity of the larynx and, if altered, prompt diagnostic and surgical intervention may be necessary.
The complex structures of the human airway vary in anatomy and physiology from birth to adulthood. The infant larynx and trachea are significantly smaller than that of an adult. At birth, the infant larynx is approximately one third the size of an adult. The glottis of the neonate measures approximately 7 mm in the sagittal plane and 4 mm in the coronal plane. The vocal cords of the newborn infant are 6-8 mm long and the vocal processes of the arytenoids extend one half of that length. In fact, the vocal process of the arytenoid takes up half the length of the vocal cord in the infant larynx, while it only takes up about ¼ of the length of the vocal cord in the adult. The posterior glottis’ transverse length is approximately 4 mm. The subglottis has a diameter of between 4.5 and 5.5 mm. These dimensions leave little margin for obstruction in the infant, unlike the adult. The narrowest portion of the airway in the older child and adult is the glottic aperture, while the narrowest part of the airway in the infant is the subglottis. A diameter of 4.0 mm is considered the lower limit of normal in a full term infant and 3.5 mm in a premature infant. Indeed, an infant with one millimeter of glottic edema will experience a 35% obstruction of the airway. In the subglottis, one millimeter of circumferential edema leads to over 60% narrowing.
The infant larynx is positioned higher in the neck than the adult larynx. The superior border of the larynx of the infant is located at about the level of the first cervical vertebrae with the cricoid positioned at about the fourth cervical vertebrae. In comparison, the adult cricoid rests about the level of the sixth cervical vertebrae. The structures of the infant larynx are more pliable and less fibrous making the infant airway more susceptible to narrowing from edema and less easily palpable.
The epiglottis is proportionally narrower than that of an adult and assumes either a tubular form or the shape of the Greek letter omega.
Embryology
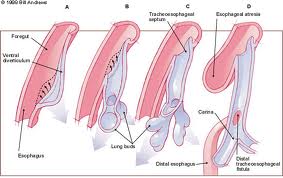
Richter recorded the identification of specific congenital laryngeal anomalies as early as 1792. In 1885, His described the appearance of the respiratory primordium from an outpouching of the cephalic portion of the developing pharynx by the third week of gestation. Indeed, the respiratory system is an outgrowth of the primitive pharynx. The development of the lower respiratory system begins at 26 days after conception as the laryngotracheal groove (also known as the respiratory primordium) at the ventral aspect of the foregut.
The laryngotracheal diverticulum becomes separated from the foregut by the tracheoesophageal folds, which fuse to become the tracheoesophageal septum. This septum divides the foregut into a ventral laryngotracheal tube and a dorsal esophagus. Failure of the tracheoesophageal folds to fuse during the fourth and fifth weeks can lead to a tracheoesophageal fistula.
The larynx develops from the fourth and fifth branchial arches. The laryngotracheal opening lies between these two arches. This primitive laryngeal aditus is altered to become a T-shaped opening by the growth of three tissue masses. One is the hypobranchial eminence. This mesodermal structure eventually becomes the epiglottis. The second and third growths are two arytenoid masses. As these masses grow between the fifth and seventh weeks, the laryngeal lumen is obliterated. Recanalization occurs by the tenth week. Failure to recanalize may result in atresia, stenosis or web formation in the larynx. The arytenoid masses are separated by an interarytenoid notch, which eventually becomes obliterated. If obliteration does not occur, a posterior laryngeal cleft can result leading to severe aspiration in the newborn.
Clinical Manifestations and Diagnosis
The clinical manifestations associated with congenital anomalies of the larynx include: 1) respiratory obstruction, 2) stridor, 3) a weakened or abnormal cry, 4) dyspnea, 5) tachypnea, 6) aspiration, or 7) episodes of cyanosis, or 8) sudden death. The clinical presentation of each lesion varies from the site being in the supraglottis, glottis, or subglottis. Although some congenital laryngeal lesions will present later in life, the majority present with symptomatology in the neonatal period or during infancy. Laryngeal lesions typically present with stridor, hoarseness, aphonia, and possibly feeding disorders. The stridor is usually inspiratory or possibly biphasic in nature, and should be differentiated from stertor, which primarily is caused by airway obstruction in the nasal or pharyngeal regions. The varied presentations of particular lesions will be discussed by region.
A child with a suspected congenital laryngeal lesion should undergo a complete history and physical exam. In the infant or child, a thorough history should be obtained which includes: history of prematurity and associated medical problems and intubation records. A premature infant who has been intubated for variable periods of time may develop acquired lesions such as subglottis stenosis, subglottic cysts, or intubation granulomas. Important characteristics of the intubation include the date of first intubation, duration, size of the endotracheal tube, number of intubations, and if any intubations were traumatic. The birth record should be reviewed to assess for any birth trauma. A history of noisy breathing and difficulty feeding should lead to suspicion of airway problems. Growth curves should be reviewed and followed to determine if the child has failure to thrive. Particularly the relationship of airway symptoms to feeding is important to elicit in the history. Suspected foreign body aspirations should be elicited and the details of changes in the baby’s cry should be discussed.
A complete head and neck exam should be performed on all patients, if possible. It begins with observing the patient for any apparent airway symptoms, which may include irritability and restlessness in an infant or dyspnea, tachypnea, cyanosis, and stridor in the infant. Voice quality in a child and crying quality in an infant should be evaluated for weakness, hoarseness, breathiness, or complete absence. Flexible fiberoptic nasopharyngolaryngoscopy may be performed at the bedside or in clinic on infants and some cooperative children if the patient is stable. Vocal cord immobility, reflux changes, immediate subglottic abnormalities, supraglottic sensation, and other glottic and supraglottic abnormalities may be detected.
Radiographic evaluation includes AP and lateral views of the neck and chest. Such films are particularly useful to help rule out any stenotic lesions of the airway. A narrowed subglottic air column suggests a diagnosis of subglottic stenosis. If inspiratory and expiratory films are difficult to obtain, airway fluoroscopy can be beneficial to view the dynamic properties of the trachea. A barium swallow can be used in cases of swallowing difficulty to delineate conditions such as posterior laryngeal clefts, tracheoesophageal fistulae, or vascular rings which may compress the trachea. The use of computerized tomography or MRI has not been proven to be beneficial in assessing the majority of congenital laryngeal etiologies, but may be useful in select cases.
If the diagnosis remains uncertain, the gold standard for diagnosis of any congenital laryngeal abnormality remains direct laryngoscopy and tracheobronchoscopy under general anesthesia. This should be performed in the operating room with an experienced anesthesiologist. It is imperative to have all of your equipment arranged and checked prior to the patient coming into the operating room. Be sure to have a wide range of bronchoscope sizes in case the airway is much smaller than you anticipated! The potential need for tracheotomy should be discussed with the patient’s family prior to endoscopy. A rigid bronchoscope or a rod lens telescope may be used to assess the airway. The important things to document during endoscopy are as follows: (1) the outer diameter of the largest bronchoscope or endotracheal tube that can be passed through a stenotic segment, if present, (2) the location/subsites (glottis, subglottis, trachea) and length of the stenosis, if present, (3) other separate sites of stenosis, (4) other airway anomalies in infants (clefts, webs, cricoarytenoid joint fixation, neoplasms, etc.), and (5) reflux changes.
Supraglottic Anomalies
Laryngomalacia
Laryngomalacia is the most common congenital laryngeal anomaly. This entity accounts for approximately 60 percent of laryngeal problems in the newborn. Boys are affected twice as often as girls. It is usually a self-limiting condition, but when severe may produce life-threatening obstructive apnea, cor pulmonale, and failure to thrive. Fatal outcomes have been described. Severe cases may require intubation or tracheotomy to secure the airway. In a series of 56 infants wit congenital airway anomalies requiring tracheotomy, 21 cases had an underlying laryngomalacia.
This condition arises from a continued immaturity of the larynx, as if the fetal stage of laryngeal development has persisted. The abnormality appears to be flaccidity or incoordination of the supralaryngeal cartilages, especially the arytenoids that is expressed when the infant is stressed by excitation with an increased respiratory rate. Stridor is typically noted in the first few weeks of life and is characterized by fluttering, high-pitched inspiratory sounds. The supraglottic structures are pulled into the lumen around a vertical axis with inspiration. The epiglottis is commonly omega shaped and the aryepiglottic folds are short. Eating difficulties and respiratory distress are rare. Sternal retractions are seen frequently with labored respiratory effort.
The diagnosis can only be made by clinical observation of the larynx during respiration. Inspection of the remainder of the respiratory tract is sometimes necessary to rule out associated secondary lesions such as innominate artery compression of the trachea or subglottic stenosis. Radiologic assessment can sometimes be a helpful adjunct if it captures the characteristic medial and inferior displacement of the arytenoid cartilage or epiglottis.
Therapy consists of confirming the diagnosis by flexible laryngoscopy and reassuring the parents that the prognosis for the child is favorable. Position changes of the infant may help alleviate the stridor as it typically worsens in the supine position. Continued vigilance by the pediatrician to be certain that the child continues to grow, feed, and breath well is important.
In the past, tracheotomy was the surgical procedure of choice for severe cases. Variot was the first to describe incising the aryepiglottic folds in severe cases of laryngomalacia. However, the first effective surgical procedure was reported in 1922, when Iglauer performed a partial epiglotticetomy. Lane was the first to publish supraglottic trimming for the treatment of laryngomalacia in 1984. A year later, Seid described using the laser to divide the AE folds. Several series since have popularized this supraglottoplasty technique with some investigators advocating the use of associated epiglottiopexy in selected cases.
Supraglottoplasty has proven successful for the correction of supraglottic obstruction and is now the surgical procedure of choice. Direct laryngoscopy and bronchoscopy should be done prior to the supraglottoplasty to ensure that no other concomitant pathology is present. The precise mechanism of obstruction should be confirmed with flexible laryngoscopy and surgical maneuvers should be directed at those sites using sharp dissection or the CO2 laser. It is important to be conservative to prevent supraglottis stenosis. Unilateral supraglottoplasty should be considered and the second side operated on only if symptoms continue. Polonovski describes a suction test where an aspiration cannula is introduced in the supraglottic inlet producing negative pressure, thereby reproducing the areas of collapse. The test is repeated after surgical excision to confirm improvement.
Results of supraglottoplasty have been impressive. Roger published the largest series comprising 115 cases. Complete regression of symptoms occurred in 53% of cases. Stridor or effort dyspnea prevailed in 36%. Seven children improved after a second procedure and 2 required eventual tracheotomy. Smaller studies have documented success rates from 77-100%. Failures may be the result of concomitant airway anomalies including pharyngomalacia. Tracheotomy may be necessary in these in these cases, however some may respond to BiPAP, obviating the need for tracheotomy.
Complications are rare and include supraglottic stenosis and swallowing problems such as aspiration. Hemorrhage, granuloma formation, posterior glottic stenosis, and cricoarytenoid joint fixation has been described.
The relationship of laryngomalacia and GERD has been described but a direct causal relationship has not been proven. Belmont found an 80% radiological prevalence of GERD in infants with LM. Phelan suggested that GERD might be a result of the high intraesophageal pressures generated. However, GERD may exacerbate LM and some authors recommend treating this condition before eventual surgery. Interestingly, however, Polonovski did not find improved respiratory symptoms with antireflux therapy.
Supraglottic Webs
Congenital webs are diaphragmatic growths of differing thickness that partially occlude the supraglottic lumen. Supraglottic webs represent less than 2 percent of congenital laryngeal webs with symptoms depending on the size and position of the web. Symptoms can include voice changes and dyspnea. Ten percent of children have other associated anomalies.
Treatment consists of surgical lysis using either the laser or sharp instrumentation, followed by dilatation. Tracheotomy should be considered if the web is large and supraglottic swelling is anticipated postoperatively.
Bifid Epiglottis
Congenital bifid epiglottis is a rare congenital laryngeal anomaly that may present with similar symptoms as laryngomalacia with inspiratory stridor and airway obstruction. The patient’s history also frequently includes episodes of cyanosis associated with feeding and occasional airway obstruction. The midline split in the epiglottis often renders the epiglottis incompetent in protecting the airway during feeding and is often drawn into the airway during inspiration. Bifid epiglottis is associated with other congenital syndromes including Pallister-Hall syndrome and polydactaly (40% of cases). Patients should undergo an endocrine evaluation because of the possible associations with hypothyroidism and hypothalamic abnormalities. Surgical management of the airway with tracheotomy may be necessary in severe cases.
Saccular Cysts
Congenital saccular cysts are unusual laryngeal anomalies that are similar in their embryologic development to laryngoceles. These lesions arise from the vestigial laryngeal structure known as the saccule. Whereas the laryngocele is filled with air and is connected to the airway, a saccular cyst is fluid filled. These cysts typically do not connect to the internal laryngeal lumen.
Desanto has classified laryngeal cysts as superior cysts (extend medially and posteriorly in the region of the ventricle) or posterior cysts (extend into the region of the false cord and AE fold). In cases of severe airway obstruction at birth, immediate intervention is warranted with either tracheotomy or intubation. Surgical management of saccular cysts can be performed endoscopically using CO2 laser and with sharp microlaryngeal instruments to marsupialize the cyst. Open surgical resection with an external laryngofissure has also been described. A high rate of recurrence is reported in several reports using endoscopic techniques and many authors stress the importance of removing the entire cyst lining.
Laryngocele
The laryngocele is a sac like structure with an internal lumen that is dilated and filled with air. It represents a dilation of the ventricular sinus of Morgagni beyond the confines of the laryngeal cartilage. They are classified as internal if they remain within the laryngeal cartilage or external if they extend through the thyrohyoid membrane. Combined lesions may also occur. Fortunately, these are rare in infants and can cause intermittent hoarseness and dyspnea that increases with crying. Diagnosis may be difficult as these can contract and may not be visualized under anesthesia. A CT may be valuable in these instances. Endoscopic and open procedures have been advocated depending on the size and location of the laryngocele.
Lymphatic and Vascular Malformations
Hemangiomas are a type of hamartoma that results in an anomalous development of blood vessels in a particular region. Hemangiomas of the supraglottic structures are rare. They can present with dyspnea, stridor, or feeding difficulties. As with hemangiomas of the subglottis, up to 30% are present at birth and most grow over the first 6-18 months. Most children, however, present in the first six months of life. Evaluation consists of careful endoscopic examination with possible CO2 laser excision, observation, tracheotomy, or open excision. As with other sites, gradual regression is expected during the first five years of life
Lymphatic malformations of the supraglottis are cystic malformations that result from abnormal development of lymphatic vessels. Lymphangiomas of the valleculla may compress the epiglottis and cause airway distress. Symptoms may include bleeding, changes in speech, dyspnea, and dysphagia. These lesions do not regress with age and treatment typically consists of careful endoscopic confirmation followed by reduction of the lesion with the CO2 laser or YAG laser. Complete excision of these lesions is often compromised by surrounding vital structures that should not be sacrificed.
Anomalous Cuneiform Cartilages
This embryonic abnormality is the result of a malformation of the lateral masses, which are derived from a portion of the sixth branchial arch. Symptoms mimic laryngomalacia with stridor, airway distress, and dyspnea on exertion. Associated anomalies including ankyloglossia and macroglossia may occur. Treatment should be tailored to symptoms. Should severe airway obstruction be present, tracheotomy and possible supraglottic laryngectomy may be necessary.
Glottic Anomalies
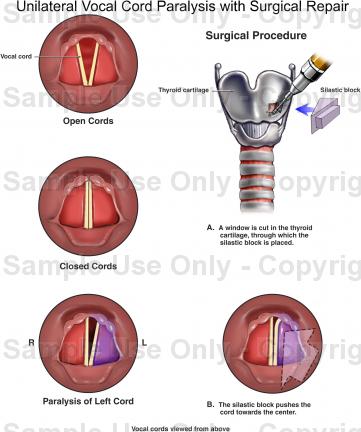
Vocal Cord Paralysis
Vocal fold paralysis has long been recognized as a significant cause of stridor and hoarseness in infants and children. It is the second most common cause of stridor in the newborn behind laryngomalacia. The frequency of vocal cord paralysis varies. Narcy quotes it as representing 23% of congenital laryngeal pathology, whereas Holinger and Fearon quote lower figures of 10% and 6.5% respectively. Some authors report unilateral paralysis to be more common and others report bilateral paralysis to be more frequent. Laryngeal paralysis may be present at birth or may manifest itself in the first month or two of life. The neurologic impairment reflects an injury to the vagus nerve. The lesion can occur anywhere from the brain through the neck into the chest and into the larynx. Many paralyses are idiopathic (cause unknown) in up to 47% of cases, however the most common causative factors include entities such as Arnold Chiari malformations, hydrocephalus, neonatal hypotonia, and multiple peripheral paralysis (myasthenia gravis). Other causes include birth trauma and cardiac anomalies. Associated laryngeal lesions such as clefts and stenosis are also commonly often found.
Any or all of the normal laryngeal functions (voice, respiration, deglutition) may be abnormal in the pediatric patient with laryngeal paralysis. The most common symptom is stridor. Ineffective cough, aspiration, recurrent pneumonia, and feeding difficulties are also commonly reported. Consistent stridor, cyanosis, and apnea are frequent. Voice and cry, however, may be normal particularly in cases of bilateral vocal cord paralysis. Hoarseness and dysphonia are common in cases of unilateral vocal fold paralysis.
The initial concern in any child with suspected laryngeal paralysis is airway stability. Extensive diagnostic evaluations are deferred until the airway is stabilized and secured. This is best achieved under the controlled setting of an operating room with appropriate airway instrumentation available. In those instances in which respiratory distress is not a primary concern, a thorough and unhurried evaluation is warranted. A careful history and physical exam is necessary. A complete past medical history including a difficult delivery, prior surgical procedures, or other congenital anomalies can provide important clues to this diagnosis.
Various methods have been used to document laryngeal paralysis. Neck films, fluoroscopy, and ultrasound have all been evaluated and are unsatisfactory in diagnosing this condition for a number of varied reasons. Chest films however are useful in diagnosing associated cardiac or pulmonary anomalies, both of which are common in cases of unilateral paralysis. Flexible laryngoscopy in the awake patient, however, is the gold standard for the diagnosis of this condition. Once the diagnosis is made, a search for the underlying cause is warranted. The entire course of the vagus nerves should be imaged. This should include CT or MR imaging. A barium swallow can provide evidence of subtle neurologic abnormalities, abnormal sensation to the larynx, and can document associated mediastinal anomalies such as vascular rings. While flexible laryngoscopy is invaluable in this diagnosis, rigid laryngoscopy and bronchoscopy is important for the identification of associated anomalies or when the cause remains unknown after a noninvasive workup has been complete. Paralysis must be differentiated from cricoarytenoid joint fixation or posterior glottic stenosis, both which can lead to a similar presentation.
Management strategies depend on the child’s underlying condition. Children with bilateral vocal fold paralysis frequently require surgical intervention. The airway is often markedly compromised and in over 50% of cases a tracheotomy is required. In cases of mild airway symptomatology with bilateral vocal fold paralysis, expectant close follow up is possible. In cases of Arnold Chiari malformation and bilateral vocal fold paralysis, many authors recommend that prior to an invasive airway procedure (tracheotomy), a VP shunt or posterior fossa decompression be performed. Some authors contend that in these cases, nasotracheal intubation for four weeks should be considered, as the potential for vocal cord return is good. Others recommend immediate tracheotomy to secure the airway prior to further procedures and testing. Once a tracheotomy is performed, serial endoscopy is performed to detect a return of function. Most authors recommend waiting at least one year prior to any irreversible lateralization procedures. EMG may provide prognostic information during this time frame. Spontaneous resolution of vocal cord paralysis is thought it occur in 48-64% of cases, with rapid improvement in many patients after correction of their hydrocephalus and ACM. Multiple lateralization procedures have been proposed for those patients who do not resolve. Surgical widening of the glottis must balance voice and airway patency issues. Older techniques such as the Woodman procedure have been abandoned. CO2 laser cordotomy and open arytenoidectomy, artenoidpexy, arytenoid separation with cartilage grafting or laser arytenoidectomy and cordectomy have been proposed as more efficacious alternatives. Decannulation rates of over 60% can be expected. Reanimation of the larynx is another form of vocal cord rehabilitation for patients with bilateral vocal cord paralysis. Phrenic to recurrent laryngeal nerve anastomosis, phrenic to PCA muscle, and omohyoid nerve muscle pedicles have all been utilized. Success rates in the pediatric population are reported to be 50%. Electrical stimulators are also being explored.
The management of unilateral vocal cord paralysis in children is usually less urgent than that of bilateral paralysis. Children adjust well to persistent unilateral vocal cord paralysis with few sequelae. A weakened cry may result but an adequate airway is the typically maintained. Lateral augmentation procedures and thyroplasty techniques are not recommended in children, as speech therapy is the mainstay in this population. Thyroplasty may play a role in older children that fail conservative measures with significant dysphonia.
Laryngeal Webs
Laryngeal webs form when there is a failure of recanalization of the larynx during embryonic development, and if it persists at birth, may cause respiratory distress. Seventy-five percent of webs occur at the level of the glottis as a membrane of differing thickness that partially occludes the lumen. The web is generally located anteriorly with a concave posterior glottic opening. Most webs are thick and fibrous with a subglottic extension.
The presentation of laryngeal webs varies with the severity and type of the web. Type I laryngeal webs involve 35% or less of the glottis. The true vocal cords are visible through the web and there is little or no subglottic extension. Symptoms include a mildly abnormal cry with some hoarseness. Respiratory distress is usually not a feature. Type II webs are anterior webs involving 35-50% of the glottis. Subglottis involvement stems more from thick anterior webbing than from cricoid abnormalities. Airway symptoms are uncommon except during infection or after intubation trauma. The voice is typically weak. Type III webs involve 50-75% of the glottis. The web is thick anteriorly and the true vocal cords may not be visualized. There may be associated cricoid anomalies. Airway symptoms are often severe and marked vocal dysfunction may occur. Type IV webs occlude 75-90% or more of the glottis. It is uniformly thick and the true vocal cord is not identifiable. The patient is aphonic and immediate airway management is required at birth.
The diagnosis of this condition is usually clear on flexible laryngoscopy, however airway films may aid in the diagnosis if subglottic or cricoid pathology is also present. Treatment of this anomaly is dependent of the type of web and symptomatology. Thin membranous type I webs which produce only minimal symptoms can be observed until age 3-4 years and then divided with either the CO2 laser or cold knife. Mitomycin-C may also be applied. Some authors employ local flaps to prevent recurrence. Type II webs can be managed by incising the web along one vocal cord and then proceeding with staged dilations or by incising the web on the opposite cord two weeks later. Keel placement through an open or endoscopic approach may be necessary and a tracheotomy is generally advised if a keel is placed. Treatment of type III and IV webs is usually delayed until the child is 3-4 years old. Most infants will have a tracheotomy in place for airway control until definitive surgery is undertaken. The standard treatment for these conditions entails a tracheotomy, laryngotomy, and keel insertion. An alternative treatment is an early single stage laryngotracheal reconstruction with submucosal resection of the abnormal cricoid, mucosal flap elevation, and rotation to the anteromedial aspect of the TVC’s. A tracheotomy may be avoided with this approach.
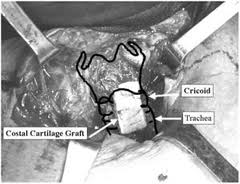
Posterior glottic webbing is rare but usually consists of a thin membranous sheet between the posterior TVC’s. Minor webs may respond to simple division and dilation, however interarytenoid webs with significant posterior glottic stenosis may require a laryngofissure, a posteriorly placed costal cartilage graft, and stenting.
Laryngeal Atresia
Laryngeal atresia is a rare congenital anomaly that is incompatible with life unless emergency measures are undertaken at birth. This condition represents the most severe end of a spectrum of diseases arising from failed recanalization of the larynx during embryogenesis. These infants only survive if there is an associated tracheoesophageal fistula or if a tracheotomy is performed immediately after birth. If a tracheoesophageal fistula is present, intubation of the esophagus may allow ventilation while airway access is being obtained. Repair of the atresia requires a formal laryngotracheal reconstruction at a later stage. This disorder is usually accompanied by a host of another anomalies including tracheoesophageal fistulae, esophageal atresia, urinary tract anomalies, and limb defects.
Congenital High Upper Airway Obstruction (CHAOS)
Congenital high airway obstruction (CHAOS) was defined by Hedrick in 1994 as upper airway obstruction that is diagnosed in utero by ultrasound, with concomitant findings of large echogenic lungs, flattened diaphragms, dilated airways distal to the obstruction, and fetal ascites or hydrops. There have been several reports of survival in infants with CHAOS who undergo the EXIT procedure (ex utero intrapartum treatment). The EXIT procedure was originally designed to treat children with large cervical masses that where diagnosed in utero and caused airway obstruction. CHAOS may not be diagnosed if a TEF is present as the fetal lung fluid is able to pass from the pulmonary system.
The EXIT procedure requires a multidisciplinary team approach. A cesarean section is performed with partial deliverance of the fetus while maintaining fetoplacental circulation. Bronchoscopy and tracheotomy can be performed expeditiously with successful outcomes reported.
Subglottic Anomalies
Subglottic Hemangiomas
Subglottic hemangiomas are congenital vascular lesions that present with symptoms ranging from minimal airway obstruction to severe, life threatening respiratory distress. Hemangiomas are present at birth only 30% of the time, with the majority of cases presenting within the first few months of life. The natural history of these lesions is similar for hemangiomas found elsewhere in the body. Typically, there is a rapid growth period that is initiated within the first few weeks or months of life and continues for 12-18 months. There is a phase where the lesion is stable and then a subsequent period of involution. Most hemangiomas involute completely by five years of age though some may not involute completely. Subglottic hemangiomas have a 2:1 female predominance.
Typically infants with subglottic hemangiomas are asymptomatic for the first few months of life and become symptomatic by the age of 3 months. Almost all are symptomatic by the age of 6 months. Stridor may initially be inspiratory but quickly becomes biphasic. The cry may be altered and the infant may have a barking cough, hoarseness, croupy symptoms, and occasional hemoptysis. Cutaneous hemangiomas occur in approximately 50% of children with subglottic hemangiomas. Thus, a child’s skin must be thoroughly examined in all cases of infant stridor.
The diagnosis of subglottic hemangioma, in most cases, can be made on clinical history, physical examination, and endoscopic appearance. Rigid endoscopy should be performed to make the definitive diagnosis, however biopsy is not always necessary. The lesion is typically a compressible symmetric bluish or reddish submucosal mass most often found in the posterior lateral subglottis. Asymmetric subglottic narrowing seen on neck films is almost pathognomic of a subglottic hemangioma as this finding is rarely seen in croup, subglottic cysts, subglottic stenosis, or RRP. MRI or CT with contrast can better delineate the lesion and assess for neck or mediastinal extension.
Numerous management options exist for subglottic hemangiomas. The decision of what therapeutic measure to take is directed at maintaining an airway while minimizing the potential long-term sequelae of the treatment itself. The treatment modalities that have been described include laser ablation using the CO2 or KTP laser, tracheotomy, external beam radiation, radioactive gold grain implantation, cryotherapy, sclerosing agents, corticosteroid therapy (systemic or intralesional), and open surgical excision. The most common intervention is CO2 laser ablation. When used conservatively, this is an appropriate treatment modality. There is, however, a risk of subsequent subglottic stenosis cause by overaggressive, circumferential lasering of large lesions. A recent report documented a 20% rate of subglottic stenosis after this treatment modality. Laser therapy is often coupled with intravenous and injectable corticosteroid therapy. Large lesions may respond solely to high dose intravenous corticosteroid therapy (1-2 mg/kg/day). Long-term side effects from this therapy may occur (growth retardation, cushingnoid appearance, and sepsis). Children with large subglottic hemangiomas causing severe airway obstruction, necessitating tracheotomy, may benefit from an open procedure. This may be performed in a single stage procedure requiring postoperative intubation for 7-10 days. A delayed procedure with delayed tracheotomy decannulation after subglottic healing may also be performed. Many authors feel the standard of care is tracheotomy with observation for involution. This method is the method by which all others should be compared.
Posterior Laryngeal Cleft
The laryngeal cleft arises at approximately 35 days of gestation from failure of rostral development of the tracheoesophageal septum. Failure of the interarytenoid tissue or cricoid cartilage to fuse in the posterior midline will result in a laryngeal cleft.
The incidence is less than 0.1% and the majority of cases are sporadic. There is a strong association with other anomalies such as tracheoesophageal fistulae. It is important to remember that 6% of patients with a TEF will have a concomitant laryngeal cleft producing continued aspirations symptoms. Laryngeal clefts are also seen in Pallister-Hall syndrome and G-syndrome. Respiratory distress is usually precipitated by feeding and is often associated with cyanosis. Voice abnormalities are often present and GERD is a major contributor to overall pulmonary compromise. Infants with these clefts often have recurrent aspiration, leading to pneumonia and death. The severity of the symptoms depends on the extent of the laryngeal cleft.
Patients suspected of having a laryngeal cleft require radiographic evaluation. Chest radiographs frequently show aspiration pneumonitis that is severe. A barium swallow is the most important diagnostic tool for showing spillover of contrast material into the trachea. Endoscopy is required to make the definitive diagnosis of a laryngeal cleft. Great care must be taken to document the relationship of the cleft to the level of the vocal cords.
Numerous classification systems exist for laryngeal clefts. Benjamin and Inglis describe type I clefts as a supraglottic, interarytenoid clefts. Type II clefts are a partial cricoid cleft. Type III clefts are a complete cricoid cleft with or without extension into the esophagus and type IV cleft are full laryngotrachealesophageal clefts.
Surgical repair must be taken in all cases of symptomatic clefts. Type I clefts can sometimes be managed nonsurgically with speech and feeding therapy aimed towards decreasing aspiration. GERD must be controlled. When conservative measures fail to prevent aspiration, endoscopic or open repair of the cleft may be possible. Type II and III clefts can be approached via an anterior laryngofissure or a lateral pharyngotomy. A tracheotomy and two-layer closure are then performed. Type IV clefts require a lateral pharyngotomy and right thoracotomy or a midline anterior approach with a median sternotomy with the patient on cardiac bypass.
The overall mortality rate of laryngeal clefts is 43%. Type IV clefts have a reported mortality rate of 93%, however the mortality rate is decreasing with advances in surgical technique.
Subglottic Stenosis
Subglottic stenosis (SGS) may be classified as either acquired or congenital. Although congenital subglottic stenosis is uncommon, accounting for 5% of all cases, it is the third most common congenital airway problem (after laryngomalacia and vocal cord paralysis). Congenital SGS is thought to be secondary to failure of the laryngeal lumen to recanalize properly during embryogenesis. SGS is considered congenital if there is no history of endotracheal intubation or other forms of laryngeal trauma.
Congenital SGS is divided histopathologically into membranous and cartilaginous types. Membranous SGS is usually circumferential and consists of fibrous soft-tissue thickening caused by increased fibrous connective tissue or hyperplastic submucous glands. It may involve the vocal folds as well. The cartilaginous type usually results from a thickened or deformed cricoid cartilage that forms an anterior subglottic shelf that extends posteriorly allowing only a small posterior opening. Other malformations can occur such as an elliptical cricoid leaving a slit-like opening or a trapped first tracheal ring. Membranous SGS is usually less severe than the cartilaginous type.
Congenital SGS is often associated with other congenital malformations. A thorough search or associated anomalies is necessary.
Subglottic stenosis is defined as a subglottic lumen 4.0 mm in diameter or less at the level of the cricoid in a full term infant. The normal newborn subglottic diameter is 4.5 – 5.5 mm and in premature neonates around 3.5 mm. A subglottic diameter of less than 3.5 mm in a premature infant is stenotic.
The severity of congenital subglottic stenosis depends on the degree of subglottic narrowing. The symptoms can range from mild with a picture of recurrent croup to severe with respiratory distress at delivery. Children with subglottic stenosis usually present with stridor and/or respiratory distress. Symptoms include irritability, restlessness, dyspnea, tachypnea and cyanosis. The stridor is typically biphasic (inspiratory and expiratory components) due to turbulent airflow through the partially obstructed airway. Frequently, children with mild congenital SGS have no symptoms until they develop an upper respiratory tract infection or under physical exertion. Any child under age one with recurrent croup should undergo endoscopy to rule out congenital SGS.
The evaluation of SGS includes a complete history and physical examination. The standard for diagnosis is rigid endoscopy under general anesthesia however airway films can demonstrate subglottic narrowing, particularly at multiple levels. These should be taken prior to undergoing evaluation in the operating room.
Historically, classification of subglottic stenosis has been a problem. Measurements were done either subjectively or by using various instruments including rigid bronchoscopes, laryngeal forceps and angioplasty catheters.
Today, there is still no universally accepted staging system for subglottic stenosis. The most commonly used system was developed by Cotton in 1984 then revised in 1989. The percentage of obstruction and anatomic location of the lesion were determined endoscopically and assigned a grade I-IV based on perceived percentage of obstruction. Although this system was successful at relating the severity of the obstruction with the prognosis for decannulation, it remained imprecise and dependent on skilled judgment. For these reasons, Myer, Conner and Cotton proposed a grading system based on endotracheal tube sizes.
The Myer-Cotton staging system is useful for mature, firm, circumferential stenosis confined to the subglottis. It describes the stenosis based on the percent relative reduction in cross-sectional area of the subglottis which is determined by differing sized endotracheal tubes. Four grades of stenosis are described with this system: grade I lesions have less than 50% obstruction, grade II lesions have 51% to 70% obstruction, grade III lesions have 71% to 99% obstruction, and grade IV lesions have no detectable lumen or complete stenosis.
The McCaffrey system classifies laryngotracheal stenosis based on the subsites involved and the length of the stenosis. Four stages are described: stage I lesions are confined to the subglottis or trachea and are less than 1cm long, stage II lesions are isolated to the subglottis and are greater then 1 cm long, stage III are subglottic/tracheal lesions not involving the glottis, and stage IV lesions involve the glottis.
Treatment of congenital SGS is tailored to the symptoms and grade of the stenosis. Symptoms are typically less severe in congenital SGS than in the acquired form. Congenital SGS also improves as the child grows, and less than half of children with this disorder will require a tracheotomy. For those children who do require surgical intervention, several options are available.
Mild stenosis (Cotton-Myer grades I and II) can usually be treated conservatively with observation. In cases that do require surgery, endoscopic techniques such as CO2 laser resection of a membranous web can be performed. Dilation has nothing to offer in the management of cartilaginous subglottic stenosis. Factors associated with failure of these endoscopic techniques include: previous attempts at endoscopic repair, circumferential scarring, loss of cartilaginous support, exposure of cartilage during laser excision leading to chondritis, severe bacterial infection, posterior inlet scarring with arytenoid fixation, combined laryngeal or tracheal stenosis or vertical scar length >1cm. Endoscopic dilation has had disappointing results and should be abandoned for congenital SGS.
Grade III or IV stenosis may require some form of open surgical procedure, as these typically are the result of a cartilaginous stenosis. Several techniques have been described.
The anterior cricoid split (ACS) procedure was originally described for a neonate who has had multiple failed extubations instead of performing a tracheotomy (Cotton and Seid, 1980). This procedure is also used for older infants and those who are have already been tracheotomized. Indications were later expanded to patients with congenital subglottic stenosis. Strict criteria for ACS have been established by Cotton and include: extubation failure on two occasions or more due to laryngeal pathology, weight >1500g, no assisted ventilation for 10 days prior to evaluation, O2 requirements <30%, no CHF for one month prior to evaluation, no acute respiratory tract infection, no antihypertensive medications ten days prior to evaluation. The procedure is performed after direct laryngoscopic and bronchoscopic confirmation of the diagnosis. All other airway pathology must be ruled-out.
A vertical midline incision is made through the cricoid cartilage and first two tracheal rings as well as the lower thyroid cartilage. This allows the cartilages to spring open and allow edematous mucosa to drain, increasing airway size. Prolene stay sutures are placed on either side of the cricoid cartilage and the skin is re-approximated after placement of a drain. The child is then left intubated, sedated and paralyzed in the ICU for 7-14 days. Cotton has guidelines for endotracheal tube sizes for stenting and for duration of stenting based on the infant’s weight.
Laryngotracheal expansion surgery involves scar division with distraction of the edges by interposition of graft material (augmentation) to widen the airway lumen. It is important to avoid removing scar, which results in a large surface area of denuded mucosa and leads to restenosis. Cotton recommends augmenting the airway with grafts when the distraction of the laryngotracheal framework must be greater than approximately 3mm. There are several techniques depending on the location and severity of the stenosis. Laryngotracheoplasty can be performed with a tracheostomy and formal stenting or by using the endotracheal tube as a stent, the latter known as a single-stage LTP (SS-LTP). There are a several stents that can be used for LTS including: endotracheal tubes, Silastic sheet rolls, Montgomery T-tubes, and laryngeal stents. Laryngeal stents include: teflon stents [Aboulker stent (short or long), ETS Poirot, Paris], and silastic stents (Montgomery stents: Boston Medical Products, Boston. The primary consideration when deciding on the type of reconstruction and stent material is to provide a safe airway and adequate support for the graft. Success of LTR among, other things, is determined by the surgical procedure, including possible need for stenting; choice of type and length of stent; and duration of stenting. Choosing the appropriate method for stenting requires considering consistency of stenosis, altered anatomy, size, location and stability of grafts when used for surgical repair and host tissue healing factors (Zalzal, 1988)
Autogenous costal cartilage is the material of choice for grafting. Many other materials have been used for grafting including auricular, hyoid and thyroid cartilage and bone. Cartilage has much less resorption over time compared to bone. Although bone provides good structural support, grafts in the airway do no bear a lot of stress or weight.
Anterior laryngofissure with anterior lumen augmentation is a technique that is good for anterior subglottic stenosis or anterior tracheal wall collapse. The lesion should not involve the glottis. Other procedures should be considered if there the cricoid cartilage is deformed or weak. Anterior grafts are made considerably larger and thicker than grafts placed posteriorly. The perichondrium is oriented to the luminal side to allow for epithelialization. The perichondrium is also a good barrier against infection. A large external flange is created to prevent the graft from prolapsing into the airway.
Laryngofissure with division of posterior cricoid lamina is indicated for patients with posterior subglottic stenosis, posterior glottic stenosis that extends to the glottis, complete or circumferential stenosis, or if there is significant cricoid deformity. Division of the anterior and posterior cricoid must be carried out for this procedure. If possible, one should avoid a complete laryngofissure to avoid damaging the anterior commissure, however this is often needed for posterior glottic involvement for access. The posterior cricoid cartilage is incised in a manner that is vertically oriented to the cartilage to allow maximal purchase for the graft. The incision is extended superiorly to the interarytenoid area and inferiorly 5 to 10 mm into the membranous trachea. The graft is elliptical in shape. It should not be too thick as it can cause swallowing difficulties and can lead to aspiration. The width of the graft is determined by the desired distraction of the cut edges of the incised posterior cricoid cartilage. 0.05 to 1.00 mm of distraction can be obtained for each year of age, up to 1 cm. It is sutured in place with absorbable suture on a small cutting needle. The knots should be buried so that they remain extraluminal to prevent development of granulation tissue. Long-term stenting is usually necessary (3-6 months).
Laryngofissure and division of posterior cricoid lamina with anterior and posterior grafts should be used for patients who have SGS similar to those above but with a significant amount of stenosis posteriorly such that grafting is necessary to maintain the adequate separation.
Once the grafts have been sutured into place in any of the above procedures, the decision must be made on whether it should be single or double-staged. Cotton and Walner (1999) recommend a double-staged procedure for patients with severe stenosis, history of reactive airway, or poor pulmonary function. This should also be considered at institutions with inadequate intensive care facilities. Double-stage procedure implies placement of stent above the tracheostomy tube instead of using an endotracheal tube as the stent (single-staged procedure). Once this decision is made, the strap muscles are closed to provide blood supply to the outer surface of the anterior graft.
The first cricotracheal resection (CTR) with thyrotracheal anastomosis was performed by Conley in 1953 in a patient undergoing surgery for chondroma of the cricoid cartilage. It was later popularized by Ogura and powers (1964) as a technique for treatment of traumatic stenosis. In the 1970s it became the treatment of choice in adults with acquired subglottic stenosis from long term intubation. Until recently, surgeons were reluctant to perform this procedure in the pediatric patients because of the risk of anastamotic dehiscence and recurrent laryngeal nerve injury, and disturbing the normal growth of the larynx. The first successful CTR performed in a child was occurred in 1978 (Savary). It wasn’t until 1993, however, that the first series of 15 pediatric patients treated with CTR for severe LTS was published. Multiple subsequent series have reported using CTR for severe LTS with good outcomes (Monnier et al., 1995, Monnier et al., 1998, Stern and Cotton, 1999).
This technique is indicated if there is severe deformity of the cricoid making grafting very likely to fail. Most say that there must be at least 10 mm of normal airway below the glottis, however Cotton states that the resection can be up to the vocal folds but to expect prolonged edema. This technique is technically difficult due to the close proximity of the vocal cords and recurrent laryngeal nerves. Stenosis less than 4 cm can be resected by laryngeal release and cervical tracheal mobilization. Stenting is not required and the tracheotomy tube can usually be removed at around 4 weeks.
The goal of management of subglottic stenosis is decannulation. Success rates are dependent on the cause of the stenosis, the number of previous failed attempts, the status of the remainder of the airway, especially the glottis and the severity of the stenosis. Cotton has reported an overall pediatric LTR success rate of 92%, 97% for Grade II, 91% for Grade III, and 72% for Grade IV.
Bailey (1988) reported results of 131 pediatric airway reconstructive procedures. He had a 92% success rate with patients who underwent laryngotracheoplasty procedures (no grafting) and 80% success with patients who underwent LTR (with grafting). He did not report on use of any grading system.
Monnier et al. (1999) has reviewed the experience with CTR at the Department of Otolaryngology at the University of Lausanne, Switzerland. 69 CTRs were performed (48 infants and children and 21 in adults). 95% and 100% of the pediatric and adult patients, all of whom had Cotton-Myer grade III or IV stenosis, were successfully decannulated. Stern and Cotton (1999) reported on 38 pediatric patients who underwent CTR for severe LTS (grade III and IV). 33 patients were successfully decannulated. Complications preventing decannulation in this study included one patient with persistent aspiration, three who restenosed, one with arytenoid prolapse, and one with recurrent laryngeal nerve injury. Overall, 94% successful decannulation has been reported in the literature when CTR is used in pediatric patients with severe LTS (Monnier, 1999).
MANIFESTATION AND DIAGNOSIS OF PEDIATRIC LARYNGOPHARYNGEAL REFLUX
Introduction:
Gastroesophageal reflux (GER) is a common physiologic condition in children; larygopharyngeal reflux (LPR) has gained increasing recognition over the past few years as a distinct pediatric condition. The former refers to retrograde flow gastric content into the esophagus, while the refluxate passes through the upper esophageal sphincter to reach the pharynx in the latter condition.
Clinical Manifestation:
Although the prevalence of LPR in children is not known, it is estimated that up to 10% of adult patients present to otolaryngologists with symptoms related to reflux. It occurs more commonly in the upright position and during daytime. Unlike GER, esophageal motility is thought to be normal in LPR. Laryngeal and pharyngeal symptoms are more common in LPR than GER, which tends to present with heartburn and/or abdominal complaints. Symptoms of LPR are non-specific, thus making accurate diagnosis difficult. Infants may present with vomiting, regurgitation, failure to thrive, irritability, chronic respiratory disorder; while children may present with dysphagia, globus sensation, otalgia, dental pain, nasal congestion, chronic cough.
Reflux has been implicated in a number of otolaryngologic conditions, including:
Chronic rhinosinusitis:
Phipps et al. reported a higher incidence of GER in patients with sinusitis and their symptomatic improvement after acid suppressive therapy. Bothwell et al. reviewed the records of 28 patients who met the criteria for endoscopic sinus surgery and 25 of them (89%) showed improvement and avoided surgery after GER treatment. Though it is possible that refluxate might reach the nasopharynx and cause inflammatory changes, there is no prospective controlled trial to support that reflux contributes to sinusitis.
Otitis media:
A recent prospective non-randomized study by Crapko et al. demonstrated that pepsin is present in 60% of middle ear effusion samples of children who underwent myringotomy for chronic otitis media with effusion (OME). A possible mechanism is reflux-induced nasopharyngeal inflammation and Eustachian tube dysfunction. Further investigation is underway to establish the relationship between LPR and otitis media in children.
Chronic cough:
Holinger and Sanders retrospectively studied 72 infants and children who had cough for at least 1 month and found that GER was present in 15% of the cases. However, there is no prospective data to date on the causal relationship between LPR and chronic cough.
Asthma:
Several studies have tried to demonstrate the relationship between asthma and GER. Debley et al. performed a cross-sectional study of 2397 adolescents and found that GER was eight times more prevalent in the asthma group than non-asthma group. The study also showed that morbidity associated with asthma, such as the number of visits to ER and clinic, is higher among those with GER. Reflux-induced bronchospasm and reduction of peak flow are possible mechanisms to explain the association.
Reflux-induced stridor:
In contrast to laryngomalacia, reflux-induced stridor is intermittent and not affected by change in position. Stridor might be a result of acid-induced laryngospasm or rapid breathing associated with esophageal irritation. Bouchard et al. reported 61 of 105 (58%) children presented with stridor and pH study-proven GERD; 83% of those improved with acid suppressive therapy. Flexible laryngoscopy is recommended to distinguish this condition from laryngomalacia.
Laryngomalacia:
Laryngomalacia is the most common cause of stridor in infants, who present with inspiratory stridor that worsens with crying or supine position. The prolapse of supraglottic structures during inspiration is thought to create negative pressure that induces upward flow of refluxate into the larynx. Incidence of GER in laryngomalacia has been reported to range from 50-80%. Direct laryngoscopy and bronchoscopy may be indicated in prolonged symptomatic cases because the incidence of a second synchronous lesion is reported to be 15-30%.
Subglottic stenosis:
Evidence linking reflux and subglottic stenosis is limited to animal studies and uncontrolled human studies. Numerous animal studies were able to demonstrate acid could induce ulceration, basilar hyperplasia, and edema of the subglottic mucosa. Yellon et al. reported that 80% of 26 children who underwent laryngotracheal reconstruction were diagnosed of GER either by barium esophagram, pH monitoring, nuclear scintiscan, or esophageal biopsy.
Diagnosis:
Because of its intermittent pattern, the diagnosis of LPR in children is often difficult. Given the limitations of the diagnostic tests discussed below, it remains controversial which test is optimal for detecting LPR.
Barium esophagram:
It is used frequently to diagnose associated anatomical anomalies such as web and stricture. However, its poor sensitivity (20-60%) as a result of short sampling time makes it less useful for diagnosing LPR.
Nuclear scintigraphy:
It has the advantage of detecting aspiration, non-acidic reflux episodes, and gastric emptying. Like barium esophagram, it only has a short sampling time and sensitivity is low (15-59 %). In addition, the correlation of scintigraphy with pH monitoring is poor.
Direct Laryngoscopy and bronchoscopy (DLB):
There is limited data evaluating DLB as a diagnostic tool in pediatric LPR. Carr et al. reported a prospective uncontrolled trial on 77 children who underwent DLB for complete airway evaluation. Endoscopic examination was graded based on laryngeal (eg. post-glottic edema, arytenoid edema) and cricotracheal (eg. cobblestoning, blunting of carina) findings. Those diagnosed with GERD were found to have significantly higher scores than those without GERD.
The subjective nature of DLB in diagnosing LPR was evaluated in Branski’s prospective randomized trial. 120 adult stroboscopic findings were graded by 5 otolaryngologists based on criteria such as edema, erythema, and pachydermia of the larynx. The study found that both inter-rater and intra-rater reliability were poor especially in arytenoids measurements. In another study, McMurray et al. also found a poor correlation between laryngoscopic findings and pH probe.
24-hour pH monitoring:
Considered the gold standard for diagnosing GER, it is one of the most commonly used techniques to document LPR. The double probe (pharyngeal, esophageal) design allows for simultaneous detection of pH change in both the hypopharynx and esophagus. Manometry has been used in the past to confirm positioning of the distal probe, which is usually 3-5 cm above the lower esophageal sphincter. Ulualp et al. recently reported a new technique using flexible laryngoscopy to guide the placement of the dual-probe.
The criteria for what constitutes a reflux episode are not standardized but usually require 1) a decrease in pH below 4 and 2) pharyngeal event following an esophageal event. The total amount of time of acid exposure in 24 hours has also been suggested as a useful criterion.
There are several limitations regarding the use of pH monitoring for LPR. It is invasive, time consuming, and generally not well tolerated by children. Brief, non-acidic, and gaseous reflux episodes might be missed by this technique. In addition, the criteria for a significant LPR episode are not well defined and vary among studies. Furthermore, pharyngeal reflux events do not correlate well with symptoms of laryngitis, as Joniau et al. pointed out in an adult study.
Multichannel Intraluminal Impedance (MII) Monitoring:
This technique measures the change in impedance between two electrodes during the passage of food bolus. Impedance, a measure of electrical resistance, decreases as bolus passes through measuring segments. It has the advantage of measuring the direction and speed of bolus, as well as detecting non-acidic and gaseous reflux episodes. Although there is no study to date using MII to diagnose pediatric LPR, preliminary results on GER are encouraging. Rosen et al. compared MII and pH monitoring in 50 children and found that sensitivity of MII (80%) is significantly higher than pH monitoring (47%) in the group treated with proton pump inhibitor.
Conclusions:
Manifestation and diagnosis of pediatric laryngopharyngeal reflux remain controversial. Despite increasing effort to establish an association between reflux and otolaryngological manifestations, conclusive evidence is lacking. In addition, well-designed controlled studies are needed to evaluate the optimal diagnostic tool for pediatric LPR.
MANAGEMENT OF THE STRIDULOUS CHILD
Introduction
Stridor is a clinical sign that is routinely encountered by pediatricians, primary care and ER physicians. Children with stridor or often referred to the “airway specialists” and it is the otolaryngologist’s task to identify the etiology of this “noisy breathing” that easily and understandably causes alarm for parents and other physicians. This chapter and the accompanying power point presentation will provide a methodical approach and framework for tackling this physical exam finding as well as a brief overview of some of the more prevalent causes of stridor.
Definitions
Stridor is a harsh sound produced by turbulent airflow through a partial obstruction. The nature of the sounds may be soft and tuneful;musical in quality. Important to remember is that stridor is characteristic of certain pathology but never diagnostic. Stertor, another term used to describe upper airway sounds is a snoring type of noise often made by nasopharyngeal or oropharyngeal obstruction, but may occasionally be created by the supraglottic larynx. Bearing these definitions in mind, it is clear to see that there is not a true separation between these two definitions therefore a wide differential diagnosis must be kept in mind.
Pathophysiology
In terms of physics, stridor can be explained as a combination of the Bernoulli principle and the law of conservation of energy. This, in essence, is how the Venturi principle is derived. This law can be applied to fluids and gases. The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. To satisfy the law of conservation of energy, the liquid/gas velocity must increase upon reaching the constriction while the pressure decreases. The decrease in pressure causes the narrowed, flexible airway of the child to close momentarily to obstruct airflow and cause stridor. In clinical terms, the “pipe” is the pediatric airway, while the “gas” is oxygen. An alternative way to comprehend this is the fact that gases normally produce equal pressure in all directions when no movement is in effect. However, as gas moves forward (linear) in a tube, it produces pressure in the forward vector while decreasing lateral pressure. When a narrowed section is encountered, the pressure in the forward vector increases while precipitously dropping in the lateral direction. Again, this drop in pressure causes the pliable pediatric airway to intermittently close yielding airway obstruction/stridor.
Anatomy
There are a number of anatomical differences between the pediatric and adult airway that render them vulnerable to compromise. In the child, the larynx is situated high in the neck with the epiglottis located behind the soft palate. The pharyngeal structures are in closer proximity compared to the adult and the hyoid bone is higher. In infants, the subglottis is the narrowest portion of the airway, thus creating a conical shape in contrast to the tubular shape in adults. This is significant because the slightest trauma or inflammation can greatly reduce airway patency. Just 1mm of edema in the pediatric tracheal airway can reduce the cross sectional area to 44% of normal! Likewise, 1mm of edema at the triangular laryngeal inlet can reduce cross sectional area to 35% of normal!
Functionally, anatomic differences associated with the infant airway create a separation between the airway and digestive tract with air movement being predominantly transnasal. As child grows the larynx descends, the pharynx becomes larger to facilitate speech production and produces a common conduit for food and air passage. In turn, this increases risk for foreign bodies, food, and gastric contents to enter the airway.
Evaluation
It is easy to become overwhelmed when presented with stridor, therefore the following pneumonic may prove useful as an initial starting point to gather important details from the history:
SPECS-R
- Severity
- Progression
- Eating difficulties
- Cyanosis
- Sleep disturbance
- Radiologic findings
- Inquire about birth history, maternal STD, and history of intubation.
In terms of physical assessment, first assess the need for emergent airway intervention (ABC’s). Initial evaluation should be noninvasive as the pediatric airway can be quite tenuous and compromise must be avoided. Indicators of severity include respiratory rate, level of consciousness/mental status, and accessory muscle use. Be cognizant of the fact that a child who stops using accessory muscles to breathe (or is not using them when you arrive at bedside) does not signify that all is well. On the contrary, this could be an ominous sign that the patient’s condition is about to plummet. During auscultation, pay attention to not only the lungs, but the neck, mouth, and nasal airway. If cyanosis is encountered in the absence of stridor, further investigation is warranted as cardiovascular, CNS, pulmonary or gastrointestinal factors may be culprits. (A brief algorithm to approaching an infant in respiratory distress is located in the power point presentation of this chapter.)
Once you’ve determined the patient is not facing imminent respiratory compromise, a more detailed and comprehensive examination may ensue. This exam should include a general assessment (weight, growth percentile, development), the nasal cavity, oral cavity and oropharynx. A heart and lung exam should always be performed. Finally, flexible fiberoptic laryngoscopy can be performed. This simple test is often the most helpful in trying to narrow down the differential diagnosis.
A formal airway evaluation in the OR is not necessary in every case. For example, in children with a history and exam (including fiberoptic), consistent with mild laryngomalacia, watchful waiting is probably all that is required. However, it is important to realize that children can have more than 1 airway issue. The flexible scope rarely allows an adequate exam of the subglottic region. In unusual or difficult cases, the ENT surgeon should have a low threshold for recommending a formal airway evaluation.
In the OR, all of the equipment (laryngoscopes, Hopkins rod-lens telescopes, and bronchoscopes) must be checked before the patient arrives to ensure they are in working condition. As a rule of thumb, a tracheostomy tray should be in room just in case an emergent surgical airway is needed. Most importantly, good communication and rapport between endoscopist and anesthesiologist is a must.
Various Etiologies of Stridor
Now, a brief review of some common causes of pediatric stridor will be examined. This approach will begin at the nose and end in the trachea. This is by no means an all inclusive list and details regarding surgical procedures of these various conditions are beyond the scope of this chapter.
Choanal Atresia (CA)
This rare disorder occurs 1 in 10,000 births with females affected more than males. There is roughly a 50/50 split between unilateral and bilateral occurrences. There are theoretically 2 types: membranous or bony; however the literature states that there are no purely membranous cases. Approximately 29% are bony and 71% mixed bony-membranous (Brown et al, Laryngoscope 1996). The pathogenesis is controversial.
Clinical Signs/Symptoms include respiratory distess/paradoxical cyanosis (i.e. cyanosis and respiratory distess that is relieved with crying), feeding difficulty and association with CHARGE syndrome:
- C- Coloboma
- H- Heart anomaly
- Atresia of choana
- R- Retarded growth
- G- Genital hypoplasia
- E- Ear anomalies and/or deafness
Clues to diagnosis include inability to pass 8 french catheter beyond 3.5 cm from nasal vestibule, and a mirror under nares that fails to fog on expiration. The flexible scope can also be quite helpful. Axial CT confirms diagnosis but in order to get the best radiographic picture it is helpful to decongest the nose and suction the secretions just before the scan.
Initially, management is conservative with the use of an oral airway or a McGovern nipple. Surgical approaches are utilized once the conservative measures fail. Each method has its pros and cons. The transpalatal approach gives better visualization, and a high success rate although this can damage the palate growth plate resulting in cross bite deformities. The transnasal method has less blood loss, and requires less procedure time; however, there is increased CSF leak and meningitis risk. Lastly, the laser (CO2, KTP, Holmium:YAG) is being utilized with good success in combination with endoscopic techniques. The operating microscope with the CO2 laser is also being employed.
Congenital Nasal Pyriform Aperture Stenosis (CNPAS)
This condition is caused by premature fusion and overgrowth of the medial nasal processes. Some believe this could represent a microform of holoprosencephaly. Associated abnormalities include a central megaincisor (60% of cases) and concomitant malfunction of pituitary/adrenal axis.
The clinical picture is very similar to CA: respiratory distress, feeding difficulty, cyclical cyanosis. The exam reveals bony obstruction of the vestibule and inability to pass a catheter/scope into the nose. A thin cut CT with emphasis on the pyriform aperture is the image modality of choice.
As with CA, management is initially conservative with use of the McGovern nipple, topical decongestants, and corticosteroids. Once conservative measures have failed, surgery is the next option. Briefly, the aperture is widened via a superior gingivolabial incision/premaxillary degloving approach to preserve the mucosa. Nasal stents are left in place 1-4 weeks.
In terms of prognosis, mild cases may resolve as the child grows but if conservative measures fail excellent long term results are usually achieved with surgery.
Retropharyngeal Abscess (RPA)
Retropharyngeal abscesses secondary to their oropharyngeal location can present as an emergent situation causing stridor. Expedient diagnosis and management are necessary.
Anatomically, the retropharyngeal space has it’s superior border at the skull base and extends inferiorly to as far as T6. It’s posterior and anterior boundaries are the prevertebral fascia and the buccopharyngeal fascia, pharyngobasilar fascia and the esophagus respectively.
Laterally, the space is bordered by the carotid sheath. Importantly, there is an anterolateral communication with the parapharyngeal space.
This entity is more prevalent in childhood with 70% of cases being in patients 6 years old or younger. The retropharyngeal space has two paramedian chains that drain the adenoids, nasopharynx, oropharynx, paranasal sinuses and possibly the middle ear. These lymph nodes are prominent in childhood but atrophy as the child approaches adolescence. When these lymph nodes suppurate, a retropharyngeal abscess may develop.
Fever, sore throat, progressive dysphagia, and drooling are all symptoms representative of retropharyngeal abscess. Patients may also present with neck stiffness and mild torticollis. In younger children, stridor may be present, the degree of which can be correlated with the size of the abscess. On physical exam, asymmetrical posterolateral pharyngeal swelling is present and may be accompanied by cervical adenopathy.
When retropharyngeal abscess is suspected CBC with differential and lateral neck films should be ordered. Lateral neck films can be up to 90% sensitive for RPA. The gold standard, however, is CT scan with contrast as this can make clarification between retropharyngeal cellulitis or abscess.
When imaging suggests cellulitis, a trial of IV antibiotics, preferably clindamycin or ampicillin-sulbactam, is a reasonable option. However, if an abscess is initially revealed on CT scan or after repeat scanning 48 hrs after antibiotics has been initiated, incision and drainage should be performed in the OR.
Laryngomalacia
This entity is the most common cause of congenital stridor. It may manifest days/weeks after birth but symptoms usually resolve by 12-18months. The stridor in laryngomalacia is believed to be caused by prolapse of supraglottic structures into laryngeal inlet.
Signs and symptoms typical of laryngomalacia include low pitched, fluttering inspiratory stridor that peaks at 6-9months of age, has positional variations, and can be exacerbated by activity (i.e. feeding, exertion). Cyanosis is rarely produced by laryngomalacia and if it is observed, suspicion for other pathology should be high.
Physical exam with awake fiberoptic laryngoscopy is needed to confirm the diagnosis. Direct laryngoscopy/bronchoscopy is sometimes needed to rule out synchronous lesions.
Management is conservative and based on the fact that this condition is self-limited. Surgical treatment (~10% of cases) comes in the form of a supraglottoplasty which is indicated for cases with severe stridor, failure to thrive, apneas, cor pulmonale, or pulmonary HTN.
Laryngeal Cysts
Laryngeal cysts are a rare form of stridor in infants. Typical symptoms include stridor, feeding difficulty, and cyanosis.
There are two types of laryngeal cysts: ductal and saccular. Ductal cysts are the most common type. The etiology of this type is obstruction of submucous glands and they can be located anywhere in larynx but most commonly in supraglottis. Saccular cysts are the least common. They are usually congenital in infants and located in the laryngeal ventricle without communication with the laryngeal lumen.
Management of laryngeal cysts is comprised of endoscopic excision or unroofing.
Congenital Laryngeal Web
Congenital laryngeal webs arise from failure of recanalization of the larynx in the embryo. They are predominantly in the anterior glottis and associated with subglottic stenosis in cases of severe webbing. Common presenting symptoms include abnormal cry and stridor.
Diagnostic endoscopy is required for diagnosis as well as ruling out other abnormalities. There appears to be and association between anterior glottic webs and velocardiofacial syndrome. There is ample evidence to support investigating for a 22q11 deletion in any child found to have a laryngeal web. Treatment ranges from simple incision for small webs to laryngofissure with stenting for severe webbing. Endoscopic laser treatment is also an option.
Posterior Laryngeal Cleft
Laryngeal clefts arise from failure of the posterior larynx to fuse (may involve trachea). Patients are usually void of stridor but present with aspiration and hoarseness. There are 4 types of laryngeal clefts and the classification correlates with the severity:
Type I-Interarytenoid cleft; superior to the glottis
Type II-Partial cricoid cleft; extends inferior to the glottis and partially through the posterior lamina of the cricoid.
Type III- Total cricoid cleft, with or without extension into the cervical tracheoesophageal wall.
Type IV- Laryngotracheoesophageal cleft extending beyond the thoracic inlet.
Diagnosis is made by demonstration of laryngeal penetration on contrast swallow, but the confirmation, as with most laryngeal pathology, is made at endoscopy.
Surgical intervention may be avoided with mild clefts and the only intervention needed may be to thicken feeds. If aspiration continues despite conservative measures then consideration of endoscopic closure is reasonable. Surgical closure utilizing lateral pharyngotomy or laryngofissure approach may be necessary in cases of extensive clefts.
Vocal Cord Paralysis
Vocal Cord Paralysis comprises 10% of congenital laryngeal lesions. It may be congenital or acquired but most often the cause is idiopathic. Various etiologies are as follows:
- Traumatic/Iatrogenic
- Obstetric/birth trauma
- Cardiac surgery
- Esophageal surgery
- Other congenital abnormalities
- Cardiac anomalies
- CNS origin (Chiari malformation)
Vocal cord paralysis can exist unilaterally or bilaterally. The key differences are briefly summarized below:
Unilateral
- Breathy voice/cry
- Mild stridor and/or dyspnea
- Aspiration
- Treatment: speech therapy. If tracheotomy needed, decannulation is usually possible as the child develops
Bilateral
- Severe stridor
- Aspiration
- Treatment: tracheotomy usually required, serial endoscopies, surgery after at least 1 year status post tracheostomy w/o improvement
Evaluation of vocal cord paralysis can be seen with fiberoptic laryngoscopy while pt is awake. Despite this, laryngotracheobronchoscopy must be performed to palpate the arytenoids and rule out congenital arytenoid fixation and exclude synchronous lesions. As further work-up, an MRI of the brain, brain stem, neck and chest are reasonable if the cause is not obvious (delineate course of vagus). FEES/MBS may be utilized in cases of aspiration.
When considering management options it is important to know that vocal cord paralysis in infants usually resolves in 6-18mos, so scheduled monitoring is reasonable for the first 2 yrs. During this time, a temporary tracheotomy may be necessary. If the cord paralysis does not resolve various surgical methods are being employed such as CO2 transverse partial cordotomy, costal cartilage grafting, and arytenoidopexy w/wo arytenoidectomy (CO2 laser or external approach).
Recurrent Respiratory Papillomatosis
Recurrent respiratory papillomatosis although rare overall, is the most common neoplasm of the larynx in children. The incidence of newly diagnosed RRP in children <15yo is 4.3/100,000. The childhood onset is often diagnosed between 2-4 yrs old with males being affected more than females, however there is no gender/ethnic difference regarding surgical frequency. The childhood onset is more aggressive compared to the adult onset with 19.7 surgeries per child (~4.4 per year).
The etiology is linked to HPV types 6 & 11 via maternal-fetal transmission.
commonly seen is the hallmark triad of progressive hoarseness, stridor, and respiratory distress. Patients most often present with dysphonia and stridor is usually the 2nd symptom to manifest as the condition worsens.
A list of current surgical and adjuvant treatments for RRP are below
Surgical
- Microlaryngoscopy with cups forceps removal
- Microdebrider
- CO2 laser
- Phono-Microsurgical
- KTP/Nd:YAG laser
- Flash scan lasers
Adjuvant
Interferon
- Indole-3-carbinol
- Photodynamic therapy
- Cidofovir
- Acyclovir
- Ribavirin
- Retinoic acid
- Mumps vaccine
- Methotrexate
- Hsp E7
Subglottic stenosis
Subglottic stenosis may be congenital or acquired:
Congenital
- Diagnosis made in absence of factors causing acquired stenosis
- Moderate-severe stenosis=Stridor at birth.
- Mild stenosis= Intermittent stridor
Acquired
- More common than congenital
- Usually more severe and difficult to manage
- Endotracheal intubation trauma=most commom cause
In subglottic stenosis, the degree of stenosis dictates the symptoms. During cases of severe stenosis, an infant may have stridor at birth whereas mild stenosis may not manifest until a URI takes place. In acquired SGS, a clue in neonates may be a failed extubation trial. Older children may successfully extubate but present later with progressive worsening respiratory distress.
Subglottic stenosis is quantified using the Myer-Cotton grading system:
- 0-50% obstruction= grade I
- 51-70% obstruction = grade II
- 71-99% obstruction = grade III
- No detectable lumen = grade IV
When evaluating SGS, stenotic portions may be visualized on plain films however, direct laryngoscopy/bronchoscopy is needed for confirmation and airway staging.
The best way to address SGS is to avoid the occurrence altogether. Preventative measures currently being used include the use of uncuffed, polyvinylchloride endotracheal tubes, smaller tubes, and nasotracheal intubation. Conservative treatment options have the primary goal of achieving decannulation (if tracheostomy present) or the prevention of a tracheostomy by means of close observation (grades I-II). Definitive surgical options include endoscopic methods employing use of lasers, anterior cricoid split, laryngotracheal reconstruction, cricotracheal resection.
Subglottic Hemangioma
Subglottic hemangiomas comprise 1.5% of all congenital laryngeal anomalies. Female occurrences predominate with a 2:1 female to male ratio. This condition is one of the most common neoplasm of infant airway.
Clinically, subglottic hemangiomas are usually asymptomatic at birth. The majority of patients present with biphasic stridor in first 6 months and cutaneous hemangiomas are present in 50% of patients at the time of diagnosis. The lesions are characterized by rapid growth that ceases at 12 months and may resolve by 5 years of age.
When diagnosing subglottic hemangiomas, biopsy is unnecessary due to the lesion’s pathognomonic appearance seen during endoscopy described as a compressible, submucosal mass with a reddish or bluish hue that is asymmetric and located in the posterior left subglottis.
The objective of treatment in dealing with subglottic hemangiomas is to preserve a stable airway while mitigating the long term sequelae of the treatment. Current treatment modalities involve tracheotomy (temporizing measure), steroids, laser excision, surgical excision, and interferon.
Vascular Causes
Congenital vascular anomalies make up 5% of stridor cases. Symptoms are caused by tracheal/bronchial external compression by:
- Innominate artery compression
- Vascular ring (double aortic arch)
- Pulmonary artery sling
- Aberrant right subclavian artery
Most common anomaly in mediastinum
A double aortic arch develops as a result of persistance of the fourth branchial arch and dorsal aortic root bilaterally. This is the most common symptomatic vascular ring. On the other hand, pulmonary artery slings are the most symptomatic of the noncircumferential anomalies. The right mainstem bronchus is affected in the majority of cases. Interesting, they are associated with the presence of complete tracheal rings.
The presentation of patients with the above conditions may be subtle or present with biphasic stridor/expiratory grunting along with chronic cough, recurrent bronchitis, pneumonia, feeding difficulty, and/or failure to thrive.
Diagnostic imaging of choice is CT with contrast or MRI, however, barium esophagram may reveal filling defects characteristic of these anomalies. Plain films are of limited value. Endoscopy allows greater assessment of the degree of compression.
Absolute indications for surgery include reflex apnea, 48hrs of failed medical management, and prolonged intubation. Relative indications include recurrent infections, exercise intolerance, dysphagia causing failure to thrive, concomitant SGS, asthma, and cystic fibrosis.
Tracheomalacia
Tracheomalacia is a congenital deformity of the tracheal rings. Patients usually present with expiratory stridor or respiratory distress. Like many airway lesions, the severity of symptoms depends on the extent of the lesion.
Diagnosis is made by flexible bronchoscopy in the awake patient. During this exam, collapse of anterior tracheal wall against membranous posterior portion of the trachea can be observed.
Treatment is rarely needed as most cases are self-limited although some cases may need temporary tracheotomy. In secondary tracheomalacia, treatment is directed at the underlying cause.
Foreign Body Aspiration
The majority of foreign body aspiration occurs in patients less than 3 years old. Aspiration of various foreign bodies are responsible for approximately 150 pediatric deaths/year in US. Choking accounts for 40% of accidental deaths in children <1yo.
Coins are the most commonly ingested object while food such as nuts and seeds are the most commonly aspirated. In older children fish and chicken bones are likely as well.
As stated previously, foreign bodies can be either ingested or aspirated. The clinical presentation can help the astute clinician determine whether ingestion or aspiration is more likely:
Esophageal
- Drooling
- Dysphagia
- Emesis
- Chest pain
Airway
- Cough
- Stridor
- Cyanosis
- Wheezing
- Asymmetric breath sounds
Plain films are important in FB assessment. PA and lateral CXR are good for radiopaque objects and can still prove useful despite lack of obvious foreign body. Rigid endoscopy is warranted when clinical suspicion is high despite “innocent/negative” films.
Unstable airway foreign bodies should be dealt with at the time of presentation. Of course, not all airway foreign bodies are emergencies that warrant operative intervention. When a patient with an airway foreign body presents in stable condition in the middle of the night or “after hours”, it may behoove the endoscopist to postpone going to the OR until the appropriate team and staff is present during the light of day. These personnel are already familiar with the equipment, pediatric anesthesiology is readily available, and the situation becomes less stressful for everyone involved. In the case of esophageal foreign bodies, it is possible to closely observe in the hospital in hopes of spontaneous passage (mid/distal esophagus). The exception is when an object such as a disc battery is ingested—this requires prompt removal in the OR.
Inflammatory Causes: Croup & Epiglottitis
Below is a chart briefly summarizing the differences between croup and epiglottitis as these are often confused.
| Croup | Epiglottis | ||
| Onset | 2yo | 1-5yo | |
| Etiology | Parainfluenza virus type 1 | H. Influenza Gram + bugs | |
| Symptoms/Signs | Barking cough, inspiratory stridor | Odynophagia, “sniff position” with mouth open | |
| Diagnostic | AP neck film=“steeple sign” | Lateral neck film=“thumb sign” |
| Treatment | Racemic epi, corticosteroid, humidified O2 | Airway established in OR, IV abx |
DISCUSSANTS’ REMARKS – Drs. Pine and Mukerji
Evaluation of Stridor in Children
Faculty Comments by Dr. Pine and Dr. Mukerji
The vital first step in evaluating any child with stridor is first to decide whether the patient requires urgent airway intervention. This decision is often made quickly and with limited information. A failure in judgment at this point places the patient at risk and puts the otolaryngologist in an untenable situation, having to later obtain an emergent airway in less than ideal circumstances with little or no equipment. If the clinical situation merits, it is safer to bring the patient to the operating room to secure the airway. Diagnostic and sometimes therapeutic endoscopy can be performed at the same time.
Fortunately, most children who present for evaluation of stridor are stable enough to undergo a complete history and physical exam. Additional lab tests and radiographs are ordered as needed. The single best test is probably flexible laryngoscopy. It allows a quick and usually excellent view of the nasal cavity, the nasopharynx, the hypopharynx and the larynx. There are small scopes (2.2mm) which can be easily passed thru an infant’s nasal cavity. If such a scope is not available, the standard sized scope can usually be passed thru the mouth to obtain a view of the larynx. I only attempt this in children with no teeth. This procedure is almost always possible at the bedside with few complications. It is important to realize that flexible laryngoscopy is not a substitute for a formal airway evaluation in the operating room. There are certainly cases where the flexible laryngoscopy exam can be normal in a child with serious airway issues. (i.e. vascular compression, tracheomalacia) One must also realize that finding one problem like laryngomalacia does not rule out another problem further down in the airway. In fact, many children have more than one airway problem. Otolaryngologists should have a low threshold for recommending a formal airway evaluation to include laryngoscopy bronchoscopy and possibly esophagoscopy.
There are a host of things that can cause or exacerbate stridor in children. Having a systematic approach to the history and exam can help narrow down the differential diagnosis. Ultimately, direct airway evaluation using both flexible and rigid techniques provide the most useful information and can often guide further testing if necessary. Even in this modern age with all sorts of fancy equipment, a host of possible tests to order, there is still no substitute for good clinical judgment.
PEDIATRIC SYNDROMIC HEARING LOSS
Introduction
An estimated 1 in 1,000 of the US population is born deaf or displays profound hearing loss early in childhood (Cotton and Myer). Of these, half are acquired prenatally while the remaining half will exhibit hearing loss as a result of hereditary-genetic factors. Approximately one-third of children with genetic hearing loss will display phenotypic characteristics of a syndrome while two-thirds will be nonsyndromic (i.e. hearing loss in the absence of other features). Whether the hearing loss is syndromic or nonsyndromic, it is of the utmost importance to identify these patients early so that the proper auditory rehabilitation and corrective measures can be implemented in time to provide the best prognosis. The discussion below will focus on syndromic forms of hearing loss as there are a plethora of syndromes that manifest an auditory phenotype.
Embryology of the Ear
Most deformities that present congenitally are often the result of some insult that occurs during the gestational development of the fetus. Knowledge of the embryology of the ear will serve as a good foundation with which to understand some of the syndromic malformations that display ear abnormalities and hearing loss.
Mesenchymal tissue from the first and second branchial arches contain the Hillocks of His which organize to form the auricle at 4 weeks gestation. The auricle is completely formed by 12 weeks with deformities and/or absence traceable as early as 7-8 weeks. Generally speaking, the earlier the insult in the developmental timeline, a more severe deformity can be expected. This holds true for most embryologic processes.
The external auditory canal (EAC) begins forming at 8 weeks when the ectoderm from the first branchial cleft joins the developing tympanic ring. The tympanic ring begins ossification at 12 weeks giving way to the bony EAC. At 28 weeks, the EAC begins to canalize producing a three layered tympanic membrane, thus abnormalities of the EAC are usually in conjunction with tympanic membrane abnormalities. Atresia occurs as a result of failure of the EAC to canalize and stenosis as a failure of complete canalization. Both atresia and stenosis can be traced to approximately 28-30 weeks gestation.
The formation of the stapes initializes ossicular development at 4 ½ weeks. The footplate begins to form at 7-9 weeks an d uninterrupted development is crucial to avoid malformations or absence of the oval window. At 12 weeks the anterior and posterior crura of the stapes become bowed and their ossification takes place between 18-24 weeks. Deformities of the stapes footplate and/or superstructure can be linked to the 12 week point on the embryonic timeline. The development of the malleus and incus begins at 5 weeks from Meckel’s cartilage of the 1st arch and Reichert’s cartilage of the second arch. A fused malleus-incus mass occurs when the middle ear mucosa fails to develop and separate the undeveloped ossicles from the tympanic cavity. The incus is the first ossicle to ossify at 15 weeks followed by the malleus at 16 weeks and is complete by 24 weeks.
The inner ear exists as the otic vesicle embryonically which appears approximately week 4. The otic vesicle then splits into a pars inferior and a pars superior (week 5) which will be the precursors of the semicircular canals (SCC) and utricle (pars inferior) as well as the cochlea and saccule (pars superior). Differentiation of the pars inferior and superior into the SCC, utricle, cochlea and saccule occur in weeks 6-7. The cochlea develops its basal turn, 1.5 turns and complete 2.5 turns by weeks 7, 8 and 10 respectively. Interruptions at this point are responsible for malformations such as Mondini’s dysplasia. During the same time period as the division of the otic vesicle into the pars superior and inferior, the acousticofacial ganglion divides into the acoustic ganglion and the facial ganglion. At week 5, the acoustic ganglion separates into a superior and inferior division with the superior division innervating the neuroepithelium of the superior portion of the otic vesicle (utricle, superior and lateral SCC). The inferior division of the acoustic ganglion will innervate the neuroepithelium of the saccule and the posterior SCC. Meanwhile, the facial ganglion will innervate the structures of the second branchial arch.
Basic genetics and definitions
In continuity with establishing a foundation for understanding the pathology of syndromes, a brief discussion of inheritance patterns as well as definitions to some frequently used terminology will be addressed.
Hereditary Patterns
Autosomal Dominant: Disorders with this mode of inheritance are usually expressed with alteration of only one gene in a gene pair. Males and females are equally affected and male-to-male transmission occurs. There is a 50% risk of an affected parents offspring being affected (male or female). Several factors may influence the clinical presentation such as decreased penetrance, variation in expressivity and age of onset.
Autosomal Recessive: Nearly one-third of mendelian disorders share this hereditary pattern. This pattern occurs when both parents are unaffected carriers. Each parent transmits either the normal or mutant gene to each of their offspring. As a result, there is a 25% risk of the children being affected. The risk of having siblings who are unaffected carriers is two-thirds. Consanguinity and reproduction among genetically isolated groups increases the risk of autosomal recessive disorders.
X-linked inheritance: X-linked recessive disorders are always expressed in males because they only have one copy of the X chromosome. In contrast, females are usually carriers since they possess two copies of the X chromosome. Females become carriers as a result of transmission from their affected fathers. Since males only receive Y chromosomes from their fathers, father- son transmission does not occur.
Mitochondrial Inheritance: All mitochondrial genes, located in the ovum at the time of conception, are inherited from the mother because sperm cells do not contribute mitochondria. conditions caused by mutations in the mitochondrial genome are described as following maternal inheritance and affect both male and female offspring.
Definition of commonly used terminology
Penetrance: This refers to the modification of traits by other genes and/or environmental factors they may render them clinically absent although the gene responsible for the trait is present.
Expressivity: The variation in expression of a gene’s phenotype. The gene is present, but may express the phenotype in a mild, moderate or severe form which will correlate with disease severity.
Pleiotropy: The multiple phenotypic effects in an affected individual as a result of the mutant gene or gene pair.
Malformation: Results when an abnormal developmental process is responsible for amorphologic defect in an organ, part of an organ or a region of the body.
Syndrome: The simultaneous presence of two or more malformations that are proven or assumed to be secondary to a single etiology.
Deformation: Occur due to extrinsic mechanical forces; not related to genetic information. An example would be plagiocephaly.
Disruption: This happens when processes such as ischemia, tissue breakdown, etc victimize a normal developmental process. Amputation of normally formed digits or limbs by free-floating amniotic bands as a result of early amnion rupture would be an example.
Sequence: A pattern of multiple defects that result from a single primary malformation. An example is Pierre Robin sequence in which micrognathia results in tongue displacement which results in cleft palate.
Association: A pattern of anomalies that occur in conjunction with one another more frequently than expected but have yet to be identified as a distinct syndrome or sequence. CHARGE association is an example (coloboma, heart anomalies, choanal atresia, retardation, genital andear anomalies).
Practical approach to the syndromal child child with hearing loss
As stated earlier, genetic hearing loss can either be syndromic or nonsyndromic. Physical exam and lab tests/investigations aim to detect those with syndromic hearing loss which account for 30% of genetically caused hearing loss. It is vital to identify cases that have an obvious genetic etiology in order to make a diagnosis and begin early and aggressive hearing rehabilitation as this affects prognosis in terms of speech and language development.
When taking an initial history for a pediatric patient with hearing loss, the physician should entertain the possibility of a genetic cause. When taking the family history, the physician should specifically inquire regarding the hearing status of the parents. Although the parents may appear to have no hearing deficits, the physician must bear in mind that one or both of the parents may be exhibiting variable expression of a mutant gene or reduced penetrance. This could especially be true of normal hearing parents who report having parents with early onset of hearing loss. Alternatively, the case may involve X-linked inheritance in which none of the parents themselves exhibit hearing loss despite a positive history in other family members. Often, this information is not volunteered and must be sought by the astute clinician.
The pregnancy and birth history are also important inquiries to make; especially the rubella status of the mother. In addition, attention should be paid to the developmental history of the patient. Isolated motor delay or global developmental delay could be clues to vestibular dysfunction or a syndrome diagnosis respectively. Also, reports of visual problems manifesting in the child could also point the physician toward a syndrome diagnosis.
A thorough physical exam is an important adjunct to the history and must not be confined only to the head and neck. Checking for birth marks on the trunk and limbs as well as limb deformities can prove helpful in trying to arrive at a syndrome diagnosis. It may be helpful to ask the parents to bring old photo albums of themselves and family members to help delineate normal familial traits from those that may be syndromic.
A Lastly, one the history and physical exam are complete, there are certain lab tests and investigations that may prove useful. Eudiometry of first degree relatives in addition to the patient, ophthalmology evaluation, serology for congenital infections (TORCHs), urinalysis (Export’s syndrome), EKG (Terrell and Lange-Nielsen syndrome), chromosome analysis and CT scans for profound or progressive hearing loss.
Syndromic Hearing Loss
Autosomal Dominant Syndromes
Waardenburg syndrome: Characterized by sensorineural hearing loss, abnormal pigmentation of the skin and hair, dystopia canthorum (eye inner canthi are displaced laterally), heterochromia iridis (iris colors do not match), and pinched nose. This syndrome occurs anywhere from 1 in 20,000 to 1 in 40,000 and accounts for 1-2% of people with profound hearing loss. The hearing loss can be unilateral or bilateral of varying severity. The syndrome has 4 subtypes classified according to the presence or absence of other abnormalities. In Type 1, every patient exhibits dystopia canthorum. The Type 2 phenotype is void of dystopia canthorum while Type 3 exists with upper extremity abnormalities in conjunction with Type 1 characteristics. Type 4 exhibits pigmentation abnormalities, Hirschsprung’s disease, plus findings shown in Type 2. Types 1 and 3 are linked to gene mutations in the PAX3 gene, while Types 2 and 4 are linked to the MITF and EDNRB genes respectively.
Branchio-oto-renal syndrome: This syndrome is estimated to be present in 2% of profoundly deaf children. The syndrome displays high penetrance although variable expressivity has been shown in families. Hearing impairment is estimated to be present in 70-93% of affected people but the age of onset ranges from early childhood to young adulthood. Likewise, there is varied severity ranging from mild to profound and the nature can be conductive, sensorineural or mixed. Common characteristics of the BOR phenotype include cup-shaped pinnae, preauricular pits, branchial cleft fistulae and bilateral renal anomalies. Other features displayed are preauricular tags, lacrimal duct stenosis, deep overbite and a long , narrow face. Inner ear anomalies like Mondini’s dysplasia and stapes fixation can also be present. The genetic etiology of this syndrome can be traced to the EYA1 gene. Based on the phenotypic anomalies, criteria have been developed for EYA1 testing: affected individuals must have at least 3 major criteria; two major criteria and at least two minor criteria; or one major criteria with one first- degree family member meeting BOR criteria (see accompanied POWERPOINT SLIDE SHOW.presentation for table of criteria).
Stickler Syndrome (STL): In addition to having sensorineural hearing loss that is progressive, these patients usually display a cleft palate, abnormal development of the epiphysis, vertebral abnormalities and osteoarthritis. There are three clinical subtypes that exist. Type 1 develops progressive myopathy, retinal detachment and vitreoretinal degeneration. Retina detachment is nonexistent in type 2 due to lack of the COL11A2 gene in the retina. In Type 3 shares eye and ear findings present in type 1 but has facial abnormalities. The absence of COL11A2 in the vitreous humor is the reason for the differing ocular phenotypes between Stickler types 1 and 3, and Stickler type 2. The genes with loci responsible for STL 1, 2 and 3 are COL2A1, COL11A1, and COL11A2 respectively.
Treacher Collins (TC): Also known as Fraceschetti-Zwahlen-Klein Syndrome or Mandibulo-Facial Dysostosis, this autosomal dominant entity is liked to mutations on chromosome 5q11 and some reports mention an association of maternal vitamin A hypersensitivity. Diagnostic criteria include microtia and malformed ears, midface hypoplasia, downslanting palpebral fissures, coloboma of outer 1/3 of lower eyelids, and micrognathia. The upper airway narrowing can be a major issue in infancy. The size of the nasopharynx is 50% smaller than normal and affected infants are more prone to OSA and SIDS. Hearing loss in this syndrome is usually conductive with a wide array of middle ear anomalies present such as monopodal stapes, ankylosed foot plate, malformed incus, cochlea and vestibule abnormalities. The EAC may be absent or stenosed. If sensorineural hearing loss is present, it usually occurs at high frequencies.
Osteogenesis imperfecta (OI): This is an autosomal dominant disorder displaying the triad of bone fragility, blue sclerae and hearing impairment. Other characteristics include triangular face, short stature, hypermobile mobile joints, cardiovascular abnormalities and skin disorders. The incidence is estimated to be 1 in 20,000 to 1 in 30,000. Causative mutations involve the COL1A1 or COL1A2 gene which regulate formation of type I collagen. Hearing loss is usually mixed and has a prevalence ranging from 26-78%. The hearing loss usually presents itself during the late 20s or early 30s. The conductive component of the hearing loss is attributed to the thickened and fixed stapes footplate, similar to what is seen in otosclerosis. The sensorineural component usually results from cochlear hair cell atrophy and atrophy of the stria vascularis. Also, anomalous bone formation in and around the cochlea may contribute to the sensorineural component of the hearing loss.
Neurofibromatosis Type II (NF 2): Bilateral vestibular schwannomas are the hallmark of this disease with a prevalence of 1 in 210,000 people. Other features include meningiomas (intracranial and spinal), ependymomas, gliomas, presenile lens opacities, and schwannomas located in the cranial, spinal and peripheral nerves. The skin can also manifest café-au-lait spots but not to the extent found in neurofibromatosis type I. This disease is caused by an NF 2 tumor-suppressor gene mutation on chromosome 22. Affected patients usually present in the 2nd and 4th decade. Up to 41% present with unilateral sensorineural hearing loss rather than bilateral sensorineural hearing loss due to the fact that this percentage of patients do not present with bilateral vestibular schwannomas. Patients can also have tinnitus, disequilibrium, headache and cranial nerve symptoms. Children < 15 years old may commonly present with skin or spinal tumors prior to the onset of hearing loss or development of vestibular schwannomas. In addition to the Manchester criteria for diagnosis (see accompanied power point presentation) patients who are suspicious for having NF 2 should undergo audiometry and MRI with gadolinium enhancement of the internal auditory canals.
Autosomal Recessive Syndromes
Usher Syndrome: Usher syndrome is the most common cause of autosomal recessive hearing loss. The incidence of Usher syndrome is approximately 3-5 per 100,000 in the general population an d 1-10% among profoundly deaf children. The syndrome has several subtypes based on severity of the deafness and the onset of retinitis pigmentosa (gradual retinal degeneration leading to decreased night vision, loss of peripheral vision, and blindness). Type 1 has severe hearing loss and vestibular dysfunction. The onset of retinitis pigmentosa is in childhood as opposed to type 2 where it begins after childhood. Mild to moderate hearing g loss characterizes type 2 along with normal vestibular function. In type 3, hearing loss is progressive as is the vestibular dysfunction. Retinitis pigmentosa can occur anytime in life.
Pendred syndrome: Characterized by hearing impairment associated with abnormal iodine metabolism. The responsible gene is SLC26A4 (PDS). This encodes a protein named pendrin which helps regulate iodine and chloride ion transport . Most patients have a euthyroid goiter which is sometimes detected at birth but often is not clinically evident until 8 years of age. Diagnosis of the thyroid abnormality used to depend on perchlorate discharge tests (indicates abnormal organification of nonorganic iodine) but this test is not specific for Pendred syndrome and the sensitivity is unknown. Instead, thyroid function tests are used. The hearing loss in this syndrome is severe and can be present at birth or progress with age. In addition, CT scans have revealed cochlear dysplasia (Mondini’s) an enlarged vestibular aqueduct or both.
Jervell and Lange-Nielsen Syndrome: This syndrome, although rare, should be suspected in a child with hearing loss and seizures of unknown origin and/or a family history of sudden death. Patients are characterized by severe-profound hearing loss and prolongation of the QT interval EKG. The syncopal episodes are due to a cardiac conduction defect which can manifest as early as the 2nd or 3rd year of life. The cardiac conduction defects can be attributed to mutations in potassium channel genes traced back to loci on the KVLQT1 and KCNE1 genes located on chromosomes 11p15.5 and 21q22 respectively.
Biotinidase Deficiency: Infants with severe biotinidase deficiency will display skin rashes, seizures, hair loss, hypotonia, emesis and acidosis in the first few months of life. This syndrome occurs because the infant lacks the enzyme responsible for proper biotin metabolism. Approximately 75% of affected infants will develop hearing loss if left untreated. The significance of this disorder is that if it is recognized, all the sequelae can be avoided with biotin supplementation.
X-Linked syndromes
Alport syndrome: As a result of the mutation in type IV collagen gene COL4A5, patients with Alport syndrome exhibit renal disorders and ocular abnormalities in addition to progressive sensorineural hearing loss. Renal disorders include glomerulonephritis, hematuria (“red diaper”), and renal failure. Early diagnosis is essential because the renal disease is usually more severe in males causing death secondary to uremia prior to 30 year old. Congenital cataracts are also common. The progressive sensorineural hearing loss mentioned earlier usually has an onset beginning in the 2nd decade of life.
Non-genetic Syndromes
Down’s syndrome: This is the most common of the chromosome abnormality syndromes typified by a wide range of abnormalities. Otolaryngologic findings are numerous in these patients and can affect every region of the head and neck. This includes small ears with overfolding of the superior helix, stenotic EAC and eustachian tube dysfunction. There is also an increased incidence of chronic ear disease in affected children due to increased incidence of upper respiratory infections, reduction of B and T cell function (immune system immaturity), and eustachian tube dysfunction. The hearing loss in DS is usually conductive secondary to the chronic middle ear disease but can also be due to ossicular chain abnormalities, especially the stapes. Upper airway obstruction and OSA are also problems encountered by children with DS due to the midface hypoplasia, and relative enlargement of the tongue, tonsils and adenoids in a constricted naso/oropharynx. Other systems affected include cardiovascular (ventricular-septal defect, tetrology of Fallot, patent ductus arteriosis), genitourinary (micropenis, low testosterone, infertility), musculoskeletal (atlanto-axial instability, short metacarpals and phalanges) and ocular (speckled iris;Brushfield spots). In terms of speech and behavior, most Down’s syndrome patients exhibit dysarthria and articulation deficits in conjunction with some degree of mental retardation (IQ 30-50).
Fetal Alcohol Syndrome (FAS): Of children born to alcoholic mothers, 30-40% suffer this syndrome. The amount of alcohol intake required to cause FAS has not been clearly established. Alcohol and its major metabolite, acetaldehyde, may be teratogenic. The alcohol induced developmental abnormalities can be the result of restriction of cell growth during critical periods. Characteristics of the syndrome include prenatal and postnatal growth deficiency, microcephaly, and mental retardation (average IQ of 63). Behavior is also affected as irritability and hyperactivity are common. Neural tube defects and seizure disorder may also be present. From an ophthalmology perspective, this syndrome causes hypoplasia of the optic nerve, increased tortuosity of the retinal vessels, severe microophthalmia and colobomas. Almost no system is guaranteed to be spared as cardiac, renal and skeletal anomalies may manifest themselves as well as malignant neoplasms of embryonal origin. Common facial dysmorphisms include narrow forehead, short palpebral fissures, ptosis of eyelids, midface hypoplasia, short nose, smooth philthrum, thin upper lip and hypoplastic mandible. In addition, cleft palate or cleft lip may exist. Ten percent of patients have hearing loss that may be either conductive or sensorineural.
Goldenhar’s Syndrome: Also referred to as facioauriculovertebral dysplasia (FAVD) and hemifacial microsomia (HFM), this disorder results from aberrant development of the first and second branchial arches. HFM is estimated to occur in 1 in 5600 live births, perhaps making it the most significant asymmetric craniofacial disorder. Otologic manifestations include microtia/anotia, preauricular tags, ossicular abnormalities, abnormal facial nerve course, and hearing loss (conductive > sensorineural). The hearing loss is predominantly conductive secondary to the abnormal development of the structures derived from the first and second branchial arches. Facial abnormalities include unilateral hypoplasia of the maxilla, malar and temporal bones in addition to mandibular ramus and condyle hypoplasia. Macrostomia or pseudomacrostomia (lateral cleft-like extension of the oral commisures), cleft lip or palate and delayed dental development. Lastly, the mastoid is poorly pneumatized and their may exist agenesis of the parotid gland or displacement of the gland. In terms of non-head and neck features, affected individuals can also have cardiac abnormalities such as coarctation of the aorta, ventricular septal defect, tetrology of Fallot, and patent ductus arteriosis. Renal ectopia and hydronephrosis can encompass the renal abnormalities. Limb deformities can be present as well as cerebral malformation and mental retardation. Ocular abnormalities include blepharoptosis, microopthalmia, epibulbar tumors, and retinal abnormalities leading to reduced visual acuity.
Rubella: Consists of a triad characterized by deafness, congenital cataracts and heart defects. This disease is caused by an RNA togavirus and is transmitted postnatally via respiratory secretion, saliva, or direct contact. Transplacental transmission is the route responsible for congenital infection which can involve more sequelae if infection is present during the first trimester. In terms of diagnosis, positive viral culture must be obtained, rubella-specific IgM antibody, or demonstration of significant rise in IgG antibody in acute (7-10days) and convalescent phase (2-3 weeks later). The virus can be cultured from blood, nasal secretions, urine, throat swab or CSF. In addition to the above listed triad, other abnormalities that may manifest are microcephaly, motor and neural retardation, hepatosplenomegaly, thrombocytopenia, encephalitis and interstitial pneumonitis. The hearing loss in rubella is typically asymmetric and sensorineural with variable severity. The 500-2000 Hz frequencies are the most commonly affected. This hearing deficit usually manifests by 5 years of age and can be an isolated finding in 22%. Approximately 25% of patients will experience a progressive form of hearing loss.
Cytomegalovirus (CMV): CMV has an incidence of 0.2%-2.3% of live births making it one of the most frequently occurring viruses worldwide and the leading cause of congenital malformations and mental retardation in developed countries. Of all the TORCH infections, CMV is the most common. Microcephaly, intrauterine growth restriction (IUGR), petechiae , encephalitis, hepatosplenomegaly, and deafness are some of the physical characteristics of a congenital CMV infection. CMV is estimated to account for 1/3 of sensorineural hearing loss in young children. Hearing impairment in CMV can be delayed (occurring months-years after birth), or fluctuating and progressive. Interesting to note, infants with petechiae and IUGR are 2-3 times more likely to have sensorineural hearing loss. Post mortem temporal bone studies on infants who died from cytomegalic inclusion disease have revealed inclusion bodies in the stria vascularis, Reissner’s membrane, saccule, utricle and semicircular canals. Endolymphatic hydrops was noted in the cochlear ducts.
Overall treatment goals in patients with syndromic hearing loss
The important fact to remember pertaining to syndromic hearing loss is that treatment of the hearing impairment is no different than treating a nonsyndromic patient with hearing loss. In other words, there isn’t a special hearing treatment for the profound sensorineural hearing loss in Usher syndrome vs. the child who is profoundly deaf at birth without a syndrome. There have been studies documenting success in use of cochlear implants in syndromes manifesting sensorineural hearing loss such as Usher, Waardenburg, and Jervell and Lange-Nielsen syndrome. Likewise, when syndromic patients have conductive hearing loss or a significant conductive component contributing to mixed hearing loss, the appropriate surgical procedures should be performed. This can range from being as simple as a BM&T for the recurrent middle ear effusions in Down’s to performing a stapedotomy/ossiculoplasty in osteogenesis imperfecta. Basically, the method of treatment should be selected to meet the individual needs of the patient to achieve the most benefit. In the end, the main purpose of arriving at a syndromic diagnosis is to identify those that will have hearing loss so that early and aggressive hearing rehabilitation can be initialized.
THE TONSILS AND ADENOIDS IN PEDIATRIC PATIENTS
Introduction
Pediatric patients are frequently seen in otolaryngology practice for complaints related to the tonsils and adenoids. In 1994 an estimated 140,000 U.S. children under the age of 15 had adenoidectomies and an estimated 286,000 had adenotonsillectomy. Although this is a decrease in the number of procedures performed per year from a peak of 1 million per year in the 1970’s, these are the most common major surgical procedures in children with a cost of an estimated half a billion dollars per year.
History
The first report of tonsillectomy was made by Celsus in 30 AD. He described scraping the tonsils and tearing them out or picking them up with a hook and excising them with a scalpel. Paul of Aegina published his description of tonsillectomy in 625 AD. In 1867 Wilhelm Meyer of Denmark reported a case of a 20 year old woman who complained of decreased hearing and nasal obstruction. He found her nasopharynx obstructed with soft tissue he called “adenoid vegetations”. For excision he constructed a ring knife which he passed through the nose and removed the adenoids. Samuel J. Crowe of Johns Hopkins published a paper in 1917 in which he described his technique for tonsillectomy in 1000 patients. He used a mouth gag still called the Crowe-Davis mouth gag today.
Embryology
The formation of the adenoids begins in the 3rd month of fetal development. This starts with glandular primordia in the posterior nasopharynx becoming associated with infiltrating lymphocytes. In the 5th month sagittal folds are formed which are the beginnings of pharyngeal crypts. The surface is covered with pseudostratified ciliated epithelium. By the 7th month of development the adenoids are fully formed.
The palatine tonsils also begin development in the 3rd month. They are derived from the ventral portions of the second pharyngeal pouches. 8-10 buds of pharyngeal squamous epithelium grow into the pharyngeal walls. The epithelium at the center of these buds dies and the initial crypts form. Lymphocytes infiltrate the crypts and then in the last trimester secondary branching occurs in the crypts and the lymphoid tissue is organized into follicles. The tubal tonsils are lymphoid collections behind the eustachian tube opening.
 The lingual tonsils begin development in the 5th month. The posterior tongue forms mucous glands, which become infiltrated with lymphoid cells. This is followed by the formation of lymphoid follicles and then, at birth, shallow crypts form, which are unbranched.
The lingual tonsils begin development in the 5th month. The posterior tongue forms mucous glands, which become infiltrated with lymphoid cells. This is followed by the formation of lymphoid follicles and then, at birth, shallow crypts form, which are unbranched.
Anatomy
 The lymphoid tissue of the nasopharynx and oropharynx is composed of the adenoids, the tubal tonsils, the lateral bands, the palatine tonsils, and the lingual tonsils. There are also lymphoid collections in the posterior pharyngeal wall and in the laryngeal ventricles. These structures form a ring of tissue named Waldeyer’s ring after the German anatomist who described them.
The lymphoid tissue of the nasopharynx and oropharynx is composed of the adenoids, the tubal tonsils, the lateral bands, the palatine tonsils, and the lingual tonsils. There are also lymphoid collections in the posterior pharyngeal wall and in the laryngeal ventricles. These structures form a ring of tissue named Waldeyer’s ring after the German anatomist who described them.
The adenoids or pharyngeal tonsil is a single mass of pyramidal tissue with its base on the posterior nasopharyngeal wall and it’s apex pointed toward the nasal septum. The surface is invaginated in a series of folds with some crypts but without the complex crypts found in the palatine tonsils. The epithelium is pseudostratified ciliated epithelium and is infiltrated by the lymphoid follicles. Blood supply is from the ascending palatine branch of the facial artery, ascending pharyngeal artery, pharyngeal branch of the internal maxillary artery, the artery of the pterygoid canal, and the ascending cervical branch of the thyrocervical trunk. Venous drainage is through the pharyngeal plexus and the pterygoid plexus flowing ultimately into the facial and internal jugular veins. Innervation is derived from the glossopharyngeal and vagus nerves. Efferent lymphatics drain to the retropharyngeal nodes and the upper deep cervical nodes.
The paired palatine tonsils sit in the tonsillar sinus limited by the palatoglossal arch anteriorly and the palatopharyngeal arch posteriorly. The superior pharyngeal constrictor muscle lies laterally. The palatine tonsils are surrounded by a fibrous capsule. Fully developed tonsils have between 10 and 30 crypts. The internal carotid artery lies approximately 2.0-2.5 cm posterolaterally to the tonsil. Blood supply is from the tonsillar and ascending palatine branches of the facial artery, the ascending pharyngeal artery, the dorsal lingual branch of the lingual artery and the palatine branch of the maxillary artery.
The tonsillar veins pierce the superior pharyngeal constrictor muscle to drain into the external palatine, pharyngeal and facial veins. Innervation is from the sphenopalatine ganglion via the lesser palatine and glossopharyngeal nerves. Efferent lymphatics drain into the upper deep cervical lymph nodes.
The lingual tonsils are located on the pharyngeal aspect of the tongue. They are lined by stratified squamous epithelium. Blood supply is from the lingual, ascending pharyngeal, and tonsillar branch of the facial arteries. Venous drainage is via the lingual system. Innervation is by the glossopharyngeal nerve. Efferent lymphatics drain to the deep cervical lymph nodes.
Function and Immunology
The tonsils and adenoids are part of the secondary immune system. They sit in the respiratory and alimentation tracts in position to be exposed to inspired or ingested antigens from air or food. Without afferent lymphatics the lymphoid nodules in these structures are exposed to antigen only in the crypts of the palatine tonsils and the folds of the adenoids where it is transported through the epithelial layer. The immunologic structure of the tonsils and adenoids is divided into four compartments: the reticular crypt epithelium, the extra follicular area, the mantle zone of the lymphoid follicle, and the germinal center of the lymphoid follicle. Membrane cells and antigen presenting cells are involved in transporting the antigen through the epithelial layer and presenting them to T-helper cells. When sufficient antigen is present this stimulates the B cells in the germinal zone of the lymphoid follicle to differentiate and produce antibodies. The tonsils and adenoids are involved in the production of mostly secretory IgA, which is transported to the surface providing local immune protection.
The effect of adenotonsillectomy on immune function is not well understood. There is some evidence that in children previously immunized orally for polio, titers dropped after adenotonsillectomy. Also, children who had previously undergone adenotonsillectomy had delayed and lowered immune response to polio vaccination based on IgA antibodies to polio virus.
Microbiology
Many pathogens can cause infection of the tonsils and adenoids. These include both organisms of the normal oropharyngeal flora, which become pathogenic, and external pathogens. Group A beta-hemolytic streptococcus is the most frequently recognized pathogen and is associated with a risk of rheumatic fever and glomerulonephritis. However, it is now recognized that a large number of pathogens can cause inflammation in the tonsils and adenoids, and that many infections are polymicrobial. The following is a list of organisms commonly cultured from the tonsils and adenoids as listed in Bailey’s Head and Neck Surgery-Otolaryngology:
- Bacteria
- Aerobic
- Group A beta-hemolytic streptococci
- Groups B, C, G streptococci
- Haemophilus influenzae (type B and non-typeable)
- Streptococcus pneumoniae
- Moraxella catarrhalis
- Staphylococcus aureus
- Haemophilus parainfluenzae
- Neisseria species
- Mycobacteria species
Anerobic
- Bacteroides species
- Peptococcus species
- Peptostreptococcus species
- Actinomyces species
Viruses
- Epstein-Barr
- Adenovirus
- Influenza A and B
- Herpes simplex
- Respiratory syncytial
- Parainfluenza
Several of these pathogens, especially Staphylococcus aureus, Moraxella catarrhalis and Hemophilus influenzae can produce beta-lactamase. In poly-microbial infections this can prevent eradication of Group A beta-hemolytic streptococci with penicillins.
Adenotonsillar Disease
The major division of adenotonsillar disease is between infection/inflammation, obstruction, and neoplasm. Acute adenoiditis symptoms include purulent rhinorrhea, nasal obstruction, fever, and sometimes otitis media. This can be difficult to differentiate from an acute upper respiratory infection but tends to have a longer and more severe course. Recurrent acute adenoiditis is 4 or more episodes of acute adenoiditis in a 6-month period with intervening periods of wellness. This can have a similar presentation as recurrent acute rhinosinusitis. Chronic adenoiditis symptoms include persistent rhinorrhea, postnasal drip, malodorous breath, and associated otitis media or extra esophageal reflux lasting at least 3 months. Acute tonsillitis symptoms are fever, sore throat, tender cervical lymphadenopathy, dysphagia, and erythematous tonsils with exudates. Recurrent acute tonsillitis is the above symptoms occurring in 4-7 separate episodes occurring in one year, 5 episodes a year for 2 years, or 3 episodes a year for 3 years. Chronic tonsillitis is associated with chronic sore throat, malodorous breath, presence of tonsilliths, peritonsillar erythema, with persistent tender cervical lymphadenopathy lasting at least 3 months.
Obstructive adenoid hyperplasia includes symptoms of chronic nasal obstruction, rhinorrhea, snoring, mouth breathing, and a hyponasal voice. Obstructive tonsillar hyperplasia is heralded by snoring, obstructive breathing asleep and awake, dysphagia, nocturnal enuresis, poor school performance, decreased attention span, and voice changes. Obstructive sleep apnea in children is clinically marked by loud snoring, apneic episodes while sleeping, daytime somnolence, behavioral problems, and enuresis. Some authors recommend polysomnography to evaluate children clinically suspected of having OSA not only to determine the presence of the disorder but also to determine which children would be at risk of postoperative complications. However, the parameters used for diagnosing OSA by polysomnography in children are a matter of debate. A respiratory disturbance index of 1 to 15 has been described as an appropriate cut off level. Also, the number of centers able to perform polysomnography in children is limited, which can cause delays in treatment for affected children.
Peritonsillar abscess is classically described as an extension of an acute episode of tonsillitis with abscess formation. More recently one author has attributed this infection to abscess formation in Weber’s salivary glands at the superior tonsillar pole. Classic symptoms are fever, sore throat, dysphagia, drooling, and trismus. On exam there is a unilateral bulge of the soft palate with the uvula deviated to the opposite side. There are an estimated 13,500 annual cases. Treatment options include needle aspiration, incision and drainage, or abscess tonsillectomy (Quinsy tonsillectomy). Needle aspiration or incision and drainage may be possible in an older cooperative child but may not be optimal for young children. In one study by Blotter et al nearly 70% of children under 6 responded to a 24 course of medical therapy. The other children underwent an abscess tonsillectomy.
Congenital tonsillar masses include teratoma, hemangioma, lymphangioma and cystic hygroma. The most common malignant neoplasm is lymphoma, usually non-Hodgkin’s lymphoma. Unilateral tonsillar enlargement which is rapid and is associated with cervical lymphadenopathy and systemic symptoms should raise suspicion for lymphoma. Adenoid hyperplasia in a teenager should also raise suspicion for lymphoma.
Medical Management
For this discussion, medical management of tonsil and adenoid disease will be discussed only briefly. The medical management of adenoid or tonsillar hyperplasia includes a course of therapy with an antibiotic effective against beta-lactamase producing organisms such as clindamycin or Augmentin. The benefits of such treatment are uncertain. Some patients with obstructive adenoid hyperplasia may respond to a prolonged course of nasal steroids. The first line treatment of acute streptococcal adenotonsillitis is still penicillin. Patients who fail to respond should undergo therapy with an antibiotic effective against beta-lactamase producing organisms and anaerobes such as clindamycin, Augmentin, or penicillin plus rifampin.
Surgical Management
Adenoidectomy
The current clinical indicators for adenoidectomy as recommended by the AAO-HNS in 2000 are:
a) Four or more episodes of recurrent purulent rhinorrhea in prior 12 months in a child <12. One episode documented by intranasal examination or diagnostic imaging.
b) Persisting symptoms of adenoiditis after 2 courses of antibiotic therapy. One course of antibiotics should be with a beta-lactamase stable antibiotic for at least 2 weeks.
c) Sleep disturbance with nasal airway obstruction persisting for at least 3 months.
d) Hyponasal or nasal speech
e) Otitis media with effusion >3 months or second set of tubes
f) Dental malocclusion or orofacial growth disturbance documented by orthodontist.
g) Cardiopulmonary complications including cor pulmonale, pulmonary hypertension, right ventricular hypertrophy associated with upper airway obstruction.
h) Otitis media with effusion over age 4.
Tonsillectomy
The current clinical indicators for tonsillectomy as recommended by the AAO-HNS in 2000 are:
a) Patient with 3 or more infections per year despite adequate medical therapy.
b) Hypertrophy causing dental malocclusion or adversely affecting orofacial growth documented by orthodontist.
c) Hypertrophy causing upper airway obstruction, severe dysphagia, sleep disorders, or cardiopulmonary complications.
d) Peritonsillar abscess unresponsive to medical management and drainage documented by surgeon, unless surgery performed during acute stage.
e) Persistent foul taste or breath due to chronic tonsillitis not responsive to medical therapy.
f) Chronic or recurrent tonsillitis associated with the streptococcal carrier state and not responding to beta-lactamase resistant antibiotics.
g) Unilateral tonsil hypertrophy presumed neoplastic.
Techniques
Adenoidectomy is performed with an adenotome, shaver blade, or with curettes. Hemostasis is usually achieved with gauze packing, electrocautery, or both. Methods of tonsillectomy include cold dissection and snare, tonsillotome, monopolar electrocautery, bipolar electrocautery, and CO2 or KTP laser. Hemostasis is obtained by gauze packing, electrocautery or both. Because of cost laser tonsillectomy is usually reserved for patients with bleeding disorders. Regardless of the method used proper operative technique is most important to avoid complications.
Indications for inpatient monitoring
Tonsillectomy and adenoidectomy are frequently performed on an outpatient basis. Recommendations for inpatient observation are age less than 3 years, history of obstructive sleep apnea, children with significant associated medical problems, neurological delay, or craniofacial abnormalities, children who live a long distance from the hospital, and those children with a questionable caregiver at home. Gerber, et al. showed a significant increase in respiratory compromise in children less than 3, those with neuromuscular disorders or chromosomal abnormalities, those with difficulty breathing during sleep, restless sleep, loud snoring with apnea, and history of URI within 4 weeks of surgery. Gabalski, et al. recommended not performing outpatient surgery for morbid obesity, sleep apnea, pickwickian syndrome, airway compromise, congenital heart disease, previous anesthesia complication, mental retardation, known coagulopathy, traveling time more than 30 minutes from the hospital, and surgeon’s suspicion of poor parental ability to care for the child in the immediate postoperative period.
Complications
The incidence of mortality from adenotonsillar surgery ranges from 1 in 16,000 to 1 in 35,000 cases. Anesthetic complications and hemorrhage cause the majority of deaths. The prevalence of hemorrhage ranges from 0.1% to 8.1%. It is divided into primary bleeding, in the first 24 hours, and secondary bleeding, around 7-10 days post operatively. Other risks include vomiting, dehydration, airway obstruction due to edema, pulmonary edema, fever, velopharyngeal insufficiency, dental injury, burns, and nasopharyngeal stenosis. Atlantoaxial subluxation can occur in patients with Down syndrome and atlantoaxial joint laxity or after adenoidectomy because of Grisel’s syndrome. This is vertebral body decalcification and laxity of the anterior transverse ligament between the atlas and the axis from inflammation or infection in the nasopharynx. Spontaneous subluxation occurs about 1 week post operatively with pain and torticollis
MANAGEMENT OF PENETRATING NECK TRAUMA
 Management of penetrating neck trauma is a controversial issue in that there are a wide variety of pathways to identify serious injuries in these patients. Controversy exists because the “gold standards” for diagnosis (arteriogram and exploratory neck surgery) are quite costly and carry with them some morbidity. This Grand Rounds examines pathophysiology of penetrating neck trauma, its traditional surgical management, and recent literature regarding less invasive strategies to identify patients who will need exploratory surgery and angiography.
Management of penetrating neck trauma is a controversial issue in that there are a wide variety of pathways to identify serious injuries in these patients. Controversy exists because the “gold standards” for diagnosis (arteriogram and exploratory neck surgery) are quite costly and carry with them some morbidity. This Grand Rounds examines pathophysiology of penetrating neck trauma, its traditional surgical management, and recent literature regarding less invasive strategies to identify patients who will need exploratory surgery and angiography.
Ballistics:
The kinetic energy of the weapon determines the damage imparted to tissue in penetrating neck trauma. The formula K=1/2mv2 applies in this situation. While the kinetic energy is directly proportional to the mass of a weapon, it is proportional to the square of the velocity of a weapon. The velocity of the weapon has a greater influence than its mass on the amount of tissue damage inflicted. Therefore, penetrating neck trauma mechanisms are most appropriately divided into two general categories: high velocity injuries and low velocity injuries. Low velocity injuries are caused by impaling objects such as glass or metal in an automobile accident, knives, or ice picks. A low velocity mechanism of injury generally leads to a straight trajectory of tissue damage, with minimal collateral damage.
High velocity injuries include bullet wounds from handguns, rifles, and shotguns. Among the high velocity weapons, most pistols are of comparatively low velocity, with a kinetic energy of less than 400 ft/lb delivered at the muzzle. In contrast, rifle bullets commonly strike with energies approaching 3000 ft/lb. Shotguns are unique in that the distance at which the gun is fired is a very important factor in the level of tissue damage. Long-range injuries (>7 yards) are characterized primarily by subcutaneous and deep fascia injuries, whereas close range injuries (<3 yards) typically create massive tissue destruction and contamination of soft tissue with the “wadding” material from the shotgun shell.
The aerodynamic properties of a bullet in flight play an important role in the amount of damage inflicted to tissues. Flying bullets are similar to spinning tops: they exhibit properties such as yaw, precession, nutation, and tumbling. These deviations from straight-line motion tend to stabilize the bullet in flight, but also can result in a larger surface area of the bullet being presented to the target producing greater amounts of tissue damage. The phenomenon of cavitation occurs with high speed bullets, especially rifle injuries. When penetration of a high velocity bullet occurs, there is rapid energy release. As the energy is absorbed by tissue, the tissue starts to flow forward and outward, simultaneously creating a vacuum that sucks in contaminants, and impacting adjacent tissue not in direct contact with a bullet In thick tissues, the cavity may be entirely concealed by small entry and exit wounds, leading to an underestimation of the amount of tissue damage.
Relevant Anatomy:
The areas of the neck that can be injured in penetrating trauma were first divided into three zones by Monson, et al in a paper from Cook County Hospital (3), based mainly on patterns of vascular injury. Zone 1 is the area from the cricoid cartilage to the clavicles. It contains the inferior trachea and esophagus, and the vessels of the root of the neck: the brachiocephalic trunk, the subclavian arteries, the common carotid arteries, the thyrocervical trunk and the corresponding veins, as well as the thoracic duct, thyroid gland, and spinal cord. Zone 2 is the area from the cricoid cartilage to the angle of the mandible. It contains the common carotid arteries, the internal and external carotid arteries, the internal jugular veins, the larynx, hypopharynx, and cranial nerves 10, 11, and 12, and the spinal cord. Zone 3 is the area from the angle of the mandible to the base of the skull. It contains the carotid arteries, the vertebral arteries, the internal jugular veins, the pharynx, and multiple cranial nerves, and the spinal cord.
Frequency of injury of neck structures:
The different zones of the neck are injured with differing frequencies. Zone 2 is the most common sight of injury followed by zones 1 and 3. McConnel and Trunkey (5) combined data from 16 papers from 1963 through 1990 to estimate the frequencies of various kinds of injuries in a total 2,495 patients. According to their findings, aerodigestive tract injuries as a group were the most frequently injured organs in the neck, with 10% of the study group having injuries in the larynx or trachea. 9.6% of patients had pharyngeal or esophageal injuries. 9.0% of patients had internal jugular vein injuries. The carotid artery was the most commonly injured artery, with injury occurring in 6.7% of patients. Interestingly, the spinal cord was injured in only 3.0% of patients in this study. Other less common areas of injury included the subclavian artery (2.2%), vertebral artery (1.3%) brachial plexus (1.9%), cranial nerves 9 and 10 (.9%) and the thoracic duct (<0.1%) (see slide 11). The average mortality in the group was 4.2%, with a higher mortality from gunshot wounds than from stab wounds. The majority of deaths were caused by exsanguination.
Signs and Symptoms of Injury:
There are many recognizable symptoms and physical findings of significant neck injury. Signs of vascular injury include hemodynamic instability (shock), profuse bleeding, evolving stroke, expanding hematoma, hemoptysis, hematemsis, unequal upper extremity pulses, and presence of a bruit or thrill over the wound.
Penetrating wounds of the larynx and trachea result in subcutaneous emphysema, hoarseness, and occasionally in respiratory distress and stridor. Patients may present with bubbles of blood emitting from an adjacent neck wound. Zone 1 tracheal injuries can produce a pneumothorax.
Esophageal injury often presents with few symptoms, making early diagnosis by physical findings alone somewhat difficult. The most important finding suggesting possible esophageal injury is a bullet trajectory near the esophagus. Neck pain and blood in the saliva or nasogastric aspirate are clues. Late findings include inflammatory findings such as fever, increasing neck pain, and odynophagia.
Signs of spinal cord injury include neck pain, hemiparesis (Brown Sequard syndrome), quadriparesis, or a “spinal shock” syndrome in which hypotension is not accompanied by tachycardia.
Surgical exposure of the neck for trauma exploration:
Surgical exposure of the neck varies based upon zone of injury and is best performed in consultation with a vascular surgeon or trauma surgeon. Exposure for injuries in left zone 1 is best achieved by left anterior thoracotomy. Left posterolateral thoracotomy can be used for wider exposure of the aortic arch, the proximal left subclavian, and the left common carotid artery. For right zone 1 injuries, a midline sternotomy is most helpful and can be extended to the right supraclavicular region with removal of the medial one third of the clavicle (4). Care must be taken to avoid injury to the vagus and phrenic nerves in these approaches. Zone 2 is best exposed by a “hockey stick” incision with the long limb parallel to the anterior border of the sternocleidomastoid muscle. The wound is opened in layers, and the sternocleidomastoid muscle is retracted laterally to expose the common, internal and external carotid arteries as well as jugular vein. Methylene blue can be injected into the pharynx to check for leaks in the esophagus.
Extension of exposure into zone 3 requires mandibulotomy or mandibular subluxation techniques to visualize the distal-most parts of the internal carotid artery. Exposure of the proximal vertebral artery requires a transverse supraclavicular incision on either side of the neck. The vertebral artery enters the foramen transversarium at C6. The zone 2 incision can be utilized to visualize the vertebral artery more distally by exposing the prevertebral fascia and sweeping the longus colli laterally and removing the costotransverse bar. The third part of the vertebral artery is found by opening the prevertebral fascia parallel to the accessory nerve and dividing the origin of the upper levator scapula and detaching the tendon of the splenius cervicis. This allows visualization of the C1-C2 interspace.
Esophageal and laryngeal evaluation are performed via barium esophagram in the stable patient followed by direct esophagoscopy and direct laryngoscopy intraoperatively. The combination of barium esophagram with direct esophagoscopy has been shown to drastically decrease the rate of missed esophageal injuries.
Management of Specific Injuries:
Data have clearly shown that injury to major neck vessels is the largest cause for mortality from penetrating neck injury. Repair of major vessels while attempting to preserve blood flow to the brain is the major task of the surgeon when vessel injury is encountered. Vascular repair is best performed by or in conjunction with a surgeon skilled in vascular surgery techniques. Repair of the common carotid artery is preferred to ligation, even if the patient is comatose as long as prograde flow in the artery is present.
Repair may require an autologous saphenous vein graft. Ligation should only be performed in comatose patients in which there is no prograde arterial flow and/or repair is technically impossible Internal carotid artery repair often requires shunt placement to ensure adequate cerebral blood flow. Thrombectomy should be attempted for proximal thrombi with no evidence of distant emboli.
The use of four vessel angiography pre-operatively has led to identification of more vertebral artery injuries. Because the risk from vertebral artery injuries is low, therapies addressing injuries to these structures should also carry low morbidity. Ligation of this vessel can be attempted, though it carries a 1-3% risk of brainstem ischemia. Interventional radiologists can often identify and embolize distal vertebral artery bleeds and bleeds from the external carotid branches. The internal jugular vein can be repaired or ligated.
Esophageal injury is important to detect and repair early, before infection complicates the patient’s course. A standard neck exploration incision can be used to expose the esophagus, followed by injection of air or methylene blue into the mouth to identify possible leaks. If the esophagus is beyond repair, a controlled fistula is established with a T-tube, or the wound is exteriorized if it is low in the neck. Injuries to the hypopharynx above the level o the arytenoid cartilages can be treated with parenteral antibiotics and without oral intake for 5 to 7 days. All other wounds should undergo two-layer watertight closure and extensive neck irrigation. Patients should be kept without oral intake for 5 to 7 days after the repair.
Tracheal injuries can usually be primarily close in two layers, with an inner layer of absorbable suture incorporating the mucosa and an outer layer of submucosal permanent suture securing cartilage to cartilage. Patients can be kept intubated for 2-3 days. When loss of tracheal tissue occurs, superior and inferior tracheal release incisions aid in re-anastomosis.
Laryngeal injury usually requires tracheotomy followed by thorough direct laryngoscopy and bronchoscopy. Some injuries, such as a dislocated arytenoid cartilage can be repaired endoscopically. Endoscopic or CT evidence of laceration of the mucous membrane, exposed cartilage, immobility of the vocal folds, or displaced or comminuted fractures of cartilage are indications for open exploration. Exploration can often be performed through the standard neck exploration incision, with or without a horizontal incision at the level of the cricothyroid membrane. The infrahyoid strap muscles are separated in the midline and are retracted laterally to expose the larynx. The thyroid cartilage is incised vertically in the midline and the endolarynx is entered through the cricothyroid membrane inferiorly, with the incision extended superiorly to the thyroid membrane. All mucosal lacerations should be meticulously closed to minimize cartilage exposure with 5-0 or 6-0 fast absorbing plain gut suture. Mucosal advancement flaps may be used to achieve complete cartilage coverage. Cartilaginous fractures are fixed with wire, nonabsorbable suture, or miniplates. The anterior margins of each true vocal cord are resutured to the thyroid cartilage followed by keel placement if the anterior commissure is devoid of epithelium. Use of laryngeal stents is rarely required in penetrating neck trauma, as the laryngeal framework is usually intact. However, multiple cartilaginous fractures that cannot be adequately stabilized can be treated with removable stents that are sutured in such a way that they move with the larynx during swallowing. Stenting increases the risk of granulation tissue and infection. Recurrent laryngeal nerve injury should be treated with reanastamosis, though synkinesis inevitably results. Cricotracheal separation should be treated with meticulous repair and fixation of the cricoid cartilage, rather than laryngotracheal reconstruction, when possible.
The Evolution of Management of Penetrating Neck Injuries:
Certain steps in the management of penetrating neck trauma have remained unchanged. These include cervical spine immobilization, establishment of an airway, and circulatory support with intravenous fluids and direct pressure for exsanguinating hemorrhage. Intubation can be attempted in the field if necessary or en route. Unsuccessful oral intubation should prompt establishment of a surgical airway as needed, even if this involves placing an endotracheal tube into a tracheal defect created by the weapon. In cases of evident laryngeal trauma, tracheotomy under local anesthesia may be preferred to orotracheal intubation to avoid further injury to the larynx. The patient’s neck is carefully examined for entry and exit wounds to help determine the trajectory of the projectile. The unstable patient is always taken to the operating room for control of hemorrhage.
Management of penetrating neck wounds was once greatly influenced by wartime experience. In such settings, without adequate adjuvant testing modalities, exploratory neck surgery was seen as the safest modality for diagnosis and treatment. A large civilian series in 1956 showed that the benefit of direct repair of arterial injuries was inversely related to the time to repair (6). This revelation led to a philosophy of immediate mandatory exploration of any neck wound penetrating the platysma, even in asymptomatic patients. The widening availability of the arteriogram modified this philosophy, as it proved to be a highly accurate tool in the diagnosis and localization of arterial injury in the neck. The four vessel arteriogram is widely viewed as second only to neck exploration for determining the nature and location of vascular neck injury. It identifies intraluminal abnormalities such as thrombi and intimal flaps as well as vessel wall compromise. Currently, interventional radiologists can also embolize bleeding vessels to control hemorrhage, particularly in zone 3 injuries. Arteriograms were used extensively for asymptomatic injuries in zones 1 and 3 (where physical exam was felt to be less reliable), and mandatory neck exploration was used for evaluation of zone 2 injuries, as advocated in a study by Monson et al in 1969. That study essentially recommended pre-operative arteriograms for zone 1 and 3 injuries in stable patients, as physical exam was felt to be of limited benefit in these areas.
A large number of studies since the 1970’s have attempted to determine the reliability of the physical exam in screening patients who should undergo further evaluation and treatment for penetrating neck injuries. Mandatory neck exploration for penetrating neck wounds was often found to lead to a negative exploration rate in excess of 50%. A review of the literature published in 1994 by McConnell et al compares several studies that use physical exam to screen patients. The review notes that in those studies in which asymptomatic patients were placed in observation groups (serial q6 hour exams by a physician), the rate of negative neck exploration was low, while the rate of false negatives was negligible. A 1991 retrospective study by Mansour et al, in which all asymptomatic patients were placed in an observation group (except for zone 1 injuries, which underwent arteriogram and esophagoscopy, since physical exam was felt to be of limited value in these patients), had 63% of its study population in an observation group (see slide 18). The entire study population only had a 1.5% mortality rate, a rate similar to or less than other studies that studies that advocated a more aggressive approach.
A study retrospective by Biffi et al (8) of 312 penetrating neck wound patients used selective management in a protocol almost identical to that of Mansour et al above. All symptomatic patients were taken to the operating room after arteriograms for zone 1 and zone 3 injury patients and esophagrams for zone 1 injury patients. Asymptomatic patients were observed unless they had injury to zone 1, in which case they generally underwent esophagram and arteriogram. The study showed a negative exploration rate of 16%, with one missed injury, an ice pick injury to the esophagus and larynx in zone 1. This patient did not undergo esophagram for the zone 1 injury. This study strengthens the argument against routine neck exploration for penetrating trauma.
All of the studies mentioned to this point have advocated routine arteriogram for asymptomatic zone 1 injuries. Advocates for this practice point to studies such as one by Flint et al (9), which reports an absence of physical findings in 32% of patients subsequently found to have major vascular injury in the setting of penetrating injury to the base of the neck. However, a 2000 study by Eddy et al (10) calls into question this notion. Eddy et al’s retrospective study combined the use of chest X-ray indicators of vascular injury (widened mediastinum, apical capping, pleural effusion, deviated trachea, deviated nasogastic tube, pneumothorax, pneumomediastinum or obscured aortic knob) with physical findings of vascular injury (as discussed previously). In a series of 184 patients with zone 1 injury, 36 patients were found to have no physical findings or chest X-ray findings consistent with vascular injury and were observed without arteriogram. These patients were discharged after 1 or 2 days of observation with an uneventful hospital course. 23 of these patients were confirmed to have no injury by arteriogram. The authors conclude that the negative predictive value of a normal physical exam and chest X-ray for zone 1 injury is 100%. They calculated a cost savings of $72,000-$90,000 if none their normal CXR and P.E. patients had undergone arteriogram. However, this is a retrospective study, and not all of the observed patients underwent arteriogram to document absence of injury. Still, it points to the value of selective testing over routine testing.
Other modalities than arteriogram and neck exploration exist to diagnose vascular injury of the neck. These include the CT scan and duplex ultrasonography. Either of these modalities can be an effective screening tool to determine which patients need arteriogram or exploration. Traditionally, computed tomography has not been used in the evaluation of penetrating neck trauma. However, CT can be used to determine the trajectory of a bullet or penetrating weapon and thus determine which structures require further workup. Gracias et al (11) performed CT scans in 23 hemodynamically stable patients out of a population of 68 patients presenting with penetrating neck trauma. They determined trajectory on the CT scan by identifying the skin entry site for each neck wound with metallic markers and following the wound tract as it coursed through multiple slices of the scan. They used findings of skin violation, subcutaneous fat stranding, soft tissue air or hematoma, vertebral fracture, contrast extravasation, and missile location to identify the tracts. Based on CT findings of trajectory, they were able to avoid the need for arteriography in 50% of studied patients who would otherwise have automatically received it by other protocols (zone 1 and zone 3 injuries) and they were able to avoid the need for endoscopy in 90% percent of patients who might otherwise have received it (zone 1 injuries). With no false negatives for injuries, this study showed tremendous cost savings in these patients. However, the study group was small, and the authors themselves recommend a larger investigation.
Duplex ultrasonography can be utilized to identify vascular injury in patients. Ultrasound should be used to evaluate in stable patients the internal carotid, common carotid, external carotid and vertebral arteries as well as the internal jugular veins. A reliable technician and radiologist should be available 24 hours a day to make this method of evaluation feasible. A small double-blinded study by Ginzburg et al (12) showed a 100% true negative rate and 100% sensitivity and 85% specificity in detecting arterial injury, using arteriogram as the gold standard. Use of ultrasonography as a screening tool for arteriogram and neck exploration could result in a significant decrease in morbidity and cost as compared to mandatory arteriogram or neck exploration.
Conclusion:
The approach to managing penetrating neck trauma has changed with the awareness that modalities such as physical exam and noninvasive studies can achieve outcomes similar to routine arteriography and neck exploration. However, arteriography and neck exploration remain the gold standard for diagnosis of life-threatening injury. While controversy abounds as to when to use noninvasive diagnostic techniques, it seems appropriate to try them in asymptomatic patients, with an awareness that injuries in zones 1 and 3 are easier to miss on clinical examination alone.
PHARYNGITIS
Pharyngitis is defined as inflammation of the mucous membranes and submucosal structures of the pharynx. It accounts for over 40 million visits by adults to medical facilities each year in the U.S. More prescriptions are written for treatment of pharyngitis than any other respiratory infection including pneumonia and otitis.
A sore throat is one of the most common chief complaints encountered by an otolaryngologist. Most have a viral infection and are self-treated with over-the-counter preparations. The majorities who seek medical attention are diagnosed by clinical evaluation and respond to treatment with antibiotics or symptomatic medication, or they resolve with time. However, the pharyngeal mucosa exhibits a brisk inflammatory response to many other agents including opportunistic bacteria, fungal overgrowth, environmental pollutants, neoplasm, granulomatous diseases, and chemical or physical irritants. A sore throat of greater than 2 weeks duration, raises the possibility of additional, more sinister diagnoses.
The discussion of pharyngitis encompasses a broad range of illnesses. This review will address primarily the infections causes of pharyngitis and touch on the more common granulomatous diseases that may involve the pharynx. Clinical manifestations of tonsillitis, adenoiditis, laryngitis and deep space neck infection overlap those of pharyngitis, however, these topics are discussed in detail in other grand rounds.
Pharyngeal Anatomy
The pharynx is the continuation of the digestive and respiratory system from the oral cavity and nose. It is a funnel-shaped fibromuscular tube, approximately 15 cm long, that is the common route for air and food. In its superior part, the pharynx receives the posterior opening of the nasal cavities called choanae. The pharynx is located posterior to the nasal and oral cavities and the larynx. The pharynx is divided into three parts: (1) the nasopharynx bounded superiorly by the skull base and choanae and inferiorly by the soft palate; (2) the oropharynx bounded superiorly by the soft palate and inferiorly by the base of tongue; and (3) the laryngopharynx or hypopharynx bounded superiorly by the base of tongue and inferiorly by the inferior border of the cricoid cartilage at approximately the level of the 6th cervical vertebrae. The widest portion of the pharynx (about 5 cm) is opposite the hyoid bone and its narrowest point is the most inferior aspect where it is continuous with the esophagus.
The pharyngeal wall is composed of five layers. From superficial to deep, they are: (1) a mucous membrane covered with pseudostratified ciliated epithelium superiorly and stratified squamous epithelium inferiorly; (2) a submucosa; (3) a fibrous layer forming the pharyngobasilar fascia, which is attached to the skull; (4) a muscular layer composed of inner longitudinal and outer circular parts; and (5) a loose connective tissue larynx forming the buccopharyngeal fascia which is continuous with the fascia covering the buccinator and pharyngeal muscles and contains the pharyngeal plexus of nerves and veins.
There are six muscles in the pharynx, three overlapping constrictor muscles and three muscles that descend from the styloid process, the cartilaginous part of the auditory tube, and the soft palate. The external circular part of the muscular layer of the wall of the pharynx is formed by the paired superior, middle, and inferior constrictor muscles.
The superior constrictor is innermost progressing to the inferior constrictor, which is outermost. All three contract involuntarily in a way that results in contraction taking place sequentially from the superior to the inferior end of the pharynx. The internal longitudinal muscles of the pharynx include the stylopharyngeus, palatopharyngeus, and the salpingopharyngeus muscle, which all function in elevated the larynx during swallowing and speaking. The salpingopharyngeus muscle originates from the cartilaginous part of the auditory tube and descends the lateral wall of the pharynx where it is covered by the salpingopharngeal fold of mucous membrane. It has the added function of opening the pharyngeal orifice of the auditory tube during swallowing.
 All the muscles of the pharynx except the stylopharyngeus are innervated by the pharyngeal plexus of nerves, which run along the lateral aspect of the pharnyx. The plexus is formed by the vagus (CNX) and glossopharyngeal (CN IX) nerves and by sympathetic branches from the superior cervical ganglion. The motor fibers in the pharngeal plexus are derived from the cranial root of CN XI (the accessory nerve), and are carried by the vagus nerve to all muscles of the pharynx and soft palate, except the stylopharyngeus (supplied by CN IX) and the tensor veli palatini (supplied by CN V3).
All the muscles of the pharynx except the stylopharyngeus are innervated by the pharyngeal plexus of nerves, which run along the lateral aspect of the pharnyx. The plexus is formed by the vagus (CNX) and glossopharyngeal (CN IX) nerves and by sympathetic branches from the superior cervical ganglion. The motor fibers in the pharngeal plexus are derived from the cranial root of CN XI (the accessory nerve), and are carried by the vagus nerve to all muscles of the pharynx and soft palate, except the stylopharyngeus (supplied by CN IX) and the tensor veli palatini (supplied by CN V3).
The blood supply to the pharynx is derived primarily from branches of the external carotid artery. These include the ascending pharyngeal artery, dorsal branches from the lingual artery, tonsillar branches of the facial artery, and palatine branches from the maxillary artery.
The retropharyngeal lymph nodes are the primary drainage site for the pharyngeal lymphatics. The nasopharynx empties to retropharyngeal lymph nodes and proceeds to the lateral pharyngeal and deep jugular nodal chains. The oropharynx drains to the superior deep cervical and jugular nodes and the hypopharynx drains to the lateral pharyngeal, deep cervical, and jugular nodes.
Evaluation
History
The chief complaint of pharyngitis is sore throat. Other local symptoms- throat scratchiness, coryza, cough, and irritation-are also common in pharyngitis, particularly in pharyngitis of viral origin. As with any symptom, the onset, duration, severity, and relieving and exacerbating factors are important to elicit. The medical history should also note the concurrence of similar symptoms within the patient’s family as well as the community at large (1). In addition, the routine complete discussion of other significant medical problems (particularly history of acquired immunodeficiency syndrome (AIDS) diabetes, and other immunodeficiency disorders), past surgeries (particularly of the head and neck), history of previous irradiation to the head and neck area, current medications, and social history including tobacco, alcohol, and intravenous drug use, sexual practices, and home environment should be elicited.
Physical Exam
A full head and neck exam should be performed on any patient with complaint of sore throat with particular attention towards the neck, oral cavity, pharynx, and larynx.
The neck exam often reveals cervical adenopathy. In addition, the thyroid should be palpated as inflammatory diseases of the thyroid gland may give patients the sensation of sore throat. The oral cavity and oropharynx should be examined for hypertrophy of lymphoid tissue, mucosal congestion, erythema, and exudate. Deviation of the uvula and anterior tonsillar pillar with erthyema may indicate peritonsillar abscess. Bulges in the posterior pharyngeal mucosa may be suggestive of other suppurative processes. Indirect laryngoscopy (IDL) should be performed on all patients. If the patient is uncooperative, flexible laryngoscopy should be done. Nasal endoscopy may reveal signs of sinusitis such as purulence at the middle meatus, frontal recess or sphenoid ostium. Postnasal drip is a well-known cause of secondary pharyngitis because of irritation. The nasopharynx may be examined for visualization of the adenoids, torus tubaris, and the fossa of osenmuller.
Etiology
I. Inflammatory
- Infectious
- Viral
- Common cold (coronavirus, rhinovirus)
- Influenza
- Herpes Simplex Virus
- Varicella Zoster Virus
- Epstein-Barr
- Bacterial
- Streptococcal (S. pyogenes, S. pneumoniae)
- Haemophilus influenzae
- Moraxella catarrhalis
- Staphylococcus aureus
- Anaerobes
- Mycoplasma pneumoniae
- Chlamydia pneumoniae
- Bordetella pertussis
- Rhinoscleroma (Kebsiella rhinoscleromatis)
- Syphilis (Treponema pallidum)
- Corynebacterium diphtheriae
- Fungal
- Candida albicans and other fungi
- Allergy
- Autoimmune (Wegener’s, Hashimoto’s, relapsing polychondritis)
- AIDS
- Sarcoidosis
- Xerostomia
- Postradiation
- Sjogren’s
- Pharmacologic
- Mouth breathing
II. Traumatic
- Intraluminal tears
- Foreign body
- Caustic or irritant ingestion
- Inhalation of irritant
- Gastroesophageal reflux
- External neck trauma
III. Neoplastic
- Pediatric (Leukemia, Lymphoma, Rhabdomyosarcina)
- Adult (Squamous cell carcinoma, Lymphoma)
- Congenital (Branchial cleft cyst; Thyroglossal duct cyst or lingual thyroid)
IV. Nutritional
- Vitamin deficiency (A, B-complex, C)
- Dehydration
V. Degenerative
- Cervical spondylosis
- Zenker’s diverticulum
- Cricopharyngeal Achalasia
VI. Miscellaneous
- Temporomandibular joint pain
- Elongated styloid (Eagle’s syndrome)
- Carotidynia
- Coronary artery disease (angina pectoris)
- Globus pharyngitis
- Esophageal spasm
- Glossopharyngeal neuralgia
- Psychiatric
- Self-mutilation
- Factitious
- Psychosomatic
Infectious causes of Pharyngitis
Viruses
The viruses that are major causes of acute respiratory disease include influenza virus, parainfluenza viruses, rhinoviruses, adenoviruses, respiratory syncytial virus, and respiratory coronaviruses. Viruses have been isolated in 12%-42% of patients with pharyngitis or tonsillitis (Huoven). The most common agents in pharyngitis are the rhinovirus and coronavirus, but adenovirus, parainfluenza viruses, and influenza viruses also have predilection for the pharynx.
Rhinovirus and coronavirus are both single stranded, positive-sense RNA picornaviruses. There are multiple serotypes of each of these viruses. They are distinguished from enteroviruses by having an optimum temperature of 33o C for in vitro replication. This temperature approximates that of the nasopharynx in the human host and may be a factor in the localization of pathologic findings at that site.
The natural course of pharyngitis due to these viruses is usually self-limited and treatment is symptomatic. Because the clinical signs and symptoms may be identical to bacterial etiologies and cultures for viruses are not usually done, it is necessary to perform the usual work-up to rule-out treatable causes.
Epstein-Barr Virus
Epstein-Barr virus (EBV) is the etiologic agent of infectious mononucleosis (IM). It is a member of the group Herpesvirus (Herpes virus 4) and is a large-enveloped, double-stranded DNA virus (Sherris). EBV selectively infects B-lymphocyte populations (Thompson). Most early infection with EBV are asymptomatic; clinically apparent EBV infection occurs most frequently in populations in which primary EBV exposure has been delayed until the second decade of life. The disease is thus seen most often in young adults. It is defined by the clinical triad of fever, lymphadenopathy, and pharyngitis combined with the transient appearance of heterophil antibodies and atypical lymphocytosis. Other clinical findings are splenomegaly (in 50%), infrequently hepatomegaly, and dermatologic finds (in 5%) including; macular, petechial, scarlatiniform, urticarial, or erythema multiforme-like rash. Symptoms may last for weeks to months. IM should be suspected if a sore throat and malaise persist despite antibiotic treatment. A white membrane covering one or both tonsils is characteristic. Hypersensitivity to ampicillin is increased in infectious mononucleosis and the antibiotic should be avoided: a severe urticaria follows its use in 90-100% of infected individuals.
Complications of IM include autoimmune hemolytic anemia, cranial nerve palsies, encephalitis, hepatitis, pericarditis and airway obstruction. Although this infection is rarely fatal, the most frequent causes of death in previously healthy individuals with primary EBV are neurologic complications, airway obstruction, and splenic rupture.
Diagnosis of IM is usually not difficult. The constellation of fever pharyngitis, and lymphadenopathy coupled with and atypical lymphocytosis and heterophil antibodies (detected by commercial tests – Monospot test) is virtually always due to primary EBV infection and require no further studies. The circulating lymphocytes are mostly T-cells reacting to the infected B lymphocytes (Thompson). Heterophil antibodies are demonstrated in 50% of children and 90-95% of adolescents and adults with mononucleosis. If IM is suspected in a young patient with negative heterophil antibodies, the presence of IgM antibodies to EBV viral capsid antigen (VCA) is diagnostic. A drop in heterophil titers indicates resolution of the acute illness. If a patient is not improving after several weeks, heterophil titers should be drawn.
Treatment of IM usually requires only supportive management. Patients should be advised to obtain adequate rest and avoid contact sports should be avoided for 6-8 weeks to avoid splenic rupture. Glucocorticoids may hasten defervescence and the resolution of pharyngitis, however, they are only indicated for certain specific complications including airway obstruction, hemolytic anemia and thrombocytopenia. They do not alter the course of neurologic complications.
Cytomegalovirus
Cytomegalovirus (Herpes virus 5) is ubiquitous, and in the developed countries approximately 50% of adults have antibodies to it. 10-15% of children are infected by CMV by the age of 5 years. Primary cytomegalovirus (CMV) infection is the illness most frequently confused with EBV-induced IM virus. About two-thirds of adults with heterophil-negative mononucleosis have CMV-induced mononucleosis. Patients with this illness are usually older than those with EBV IM. Fever and malaise seem to be the predominant presenting symptoms with pharyngitis and lymphadenopathy being less common. The diagnosis is made by isolating CMV from the blood or showing a 4 fold rise or greater in antibody titer to CMV. CMV is a common infection in patients with HIV. The pharynx may be involved, but esophagitis is more common.
Herpes Simplex Virus
The term herpes (from the Greek herpein, “to creep”) and the clinical description of cold sores date back to Hippocrates. Two distinct epidemiologic and antigenic types of herpes simplex virus (HSV) exist (HSV-1 and HSV-2). Although both types can be involved in infections of the upper aerodigestive tract, infection with HSV-1 is usually “above the waist” while HSV-2 usually involves areas “below the waist”. HSV infection occurs in both primary and recurrent forms. It is transferred through directed contact with mucus or saliva.
The clinical manifestations and course of HSV depend on the anatomic site of the infection, the age and immune status of the host. First episodes of HSV disease, especially primary infections (i.e. first infections in which the host lacks HSV antibodies in acute-phase serum), are frequently accompanied by systemic signs and symptoms, involve both mucosal and extramucosal sites, and have a longer duration of symptoms, a longer time during which virus is isolated from lesions, and higher rate of complications than recurrent episodes of disease. Gingivostomatitis and pharyngitis are the most frequent clinical manifestations of first-episode HSV-1 infection. These infections are usually seen in children and young adults. Clinical symptoms and signs include fever, malaise, myalgias, anorexia, irritability, and cervical adenopathy, which may last from 3- 14 days. Physical exam usually reveals grouped or single vesicular lesions on an erythematous base involving the buccal mucosa and hard and soft palate that become pustular and coalesce to form single or multiple ulcers. HSV of the pharynx usually results in exudative or ulcerative lesions of the posterior pharynx and/or tonsillar pillars.
The acute illness evolves over 7-10 days, followed by rapid regression of symptoms and resolution of the lesions. On mucosal surfaces the lesions reepithelialize directly. No substantial evidence suggests that reactivation of oral-labial HSV infection is associated with symptomatic recurrent pharyngitis.
In immunosuppressed patients infection may extend deep into mucosal and submucosal layers. Friability, necrosis, bleeding, severe pain, and inability to eat or drink may result. Persistent ulcerative HSV infections are among the most common infections in patients with AIDS. These patients should be treated aggressively with intravenous acyclovir to prevent disseminated disease.
HSV can be isolated from almost all lesions. Laboratory confirmation of HSV infection is best performed by isolation of the virus in tissue culture or demonstration of HSV antigens in scrapings from lesions. Identification can be made in a variety of cell culture systems within 48 hours after inoculation. Spin-amplified culture with subsequent staining for HSV antigen has shortened the time to identification to less than 24 hours. HSV PCR techniques may be more rapid and sensitive than viral isolation.
Recommendations for treatment of HSV infections are based studies looking at herpes labialis. Those include treatment with acyclovir, 400 mg PO 5 times daily for 10 days and equally affective is valacyclovir, 1000 mg PO bid for 10 days. In one randomized controlled trial in patients with recurrent orofacial HSV, acyclovir, 400 mg PO 5 times per day for 5 days taken early in the attack led to a reduction in duration of symptoms from 12.5 to 8.1 days. More recent studies reveal reduction in recurrent disease from 36% to 19% of patients over a year who took acyclovir, 400 mg po bid every day.
Measles
The measles virus is classified in the paramyxovirus family, genus Morbillivirus. It contains linear, negative-sense, single-stranded RNA. The highest attack rates have been in childhood, usually sparing infants less than 6 months of age because of the presence of circulating maternal antibodies. Decline in reported cases over the past several decades has been attributed to increased immunization coverage. In the first half of 1990 there were 13,787 reported cases in the U.S. compared to 167 cases in 1993 during the same months. Many cases today are due to one-dose vaccine failures or groups who do not accept immunization.
The typical illness usually begins 9-11 days after exposure, with cough coryza, conjunctivitis, and fever. One to three days after onset, pinpoint gray-white spots surrounded by erythema appear on mucous membranes. This sign, called Koplik’s spots, is usually most noticeable over the buccal mucosa. Within a day of these findings, patients develop the typical measles rash, which is maculopapular and begins on the head and progresses to the trunk and extremities. The rash persists for 3-5 days and then fades. Cervical lymphadenopathy is not uncommon.
Diagnosis is made clinically in most cases. The virus can be isolated from the oropharynx and urine and grown in cell cultures, producing multinucleated giant cells as can be seen in affected tissue from the patient. Serologic studies of acute and convalescent serum samples may also be performed.
Measles is usually self-limited. Patients should be followed closely, however, to watch for signs of bacterial superinfection such as acute otitis media, sinusitis, pneumonia, mastoiditis, and sepsis. Other complications include encephalitis, acute thrombocytopenic purpura, and acute appendicitis.
Prevention of measles involves vaccination of infants with live, attenuated measles vaccine. This vaccine should be administered after the first year of life (13-15 mo) usually as a trivalent vaccine with mumps and rubella.
Human Immunodeficiency Virus
Pharyngitis in patients with human immunodeficiency virus (HIV) or AIDS is usually due to opportunistic infections such as HSV, CMV, and Candida. However, direct affects of the virus on lymphoepithelial tissues of the pharynx occur. Viral particles have been documented in the pharyngeal epithelium and tonsils.
Bacteria
Streptococci
The genus Streptococcus comprises species of Gram-positive spherical or oval cocci that tend to be arranged in chains. These bacteria form a significant portion of the indigenous microflora of humans and animals; most are found in the oral cavity and nasopharynx. Streptococcus species are classified based on their type of hemolysis (either alpha or beta). Beta-hemolytic streptococci are and further subdivided into Lancefield groups based on cell membrane carbohydrates. Lancefield group A beta hemolytic streptococcus is the most important of the pathogens causing pharyngitis. The role of other beta-hemolytic streptococci (Groups B, C, F, and G) in causing pharyngitis is yet unresolved.
Group A streptococcus is the most common bacterial cause of acute pharyngitis followed by Streptococcus pneumonia, and group C streptococci. Pharyngitis caused by group A streptococcus should always be treated; however, there has been no scientific evidence that treatment of non-group-A streptococci is beneficial. The rational for treating group A infections are as follows: (1) relief from symptoms related to the infection – the main reason patients seek medical care; (2) treatment has led to a decrease in cases of rheumatic fever, (3) treatment can prevent suppurative sequelae, and (4) also prevents the further spread of the group A streptococcus in the community.
The typical presentation of Streptococcal pharyngitis is usually indistinguishable from non-streptococcal pharyngitis (usually viral) including sore throat, erythema of involved tissues with or without purulent exudate. Thus unless the physician is able to confidently exclude the diagnosis of streptococcal pharyngitis on epidemiological and clinical ground, a laboratory test should be performed to determine whether group A streptococci are present in the pharynx.
Methods to detect group A streptococcus include rapid antigen detection tests (RADTs) for the direct identification of group A streptococci from the pharynx, the overnight slide-culture test using a bacitracin disk, and the regular blood agar culture. Several rapid antigen detection kits for group A streptococci are available. The time required to obtain final results with these assays is usually about 10 minutes. These tests are highly specific but their sensitivity may be sub-optimal. Swabs of both tonsils and the posterior pharynx should be taken.
The slide-culture test and the regular blood agar culture, require overnight incubation. In culture techniques, bacitracin-susceptible beta-hemolytic colonies found after incubation suggest the presence of group A streptococcus. This result, however, should be further confirmed by agglutination, using antisera for group A, B, C, D, F, and G streptococci, to avoid false-positive answers. Thus, the final result usually takes at least 2-3 days.
In the case of group A streptococcus, penicillin V for 10 days is the drug of choice. A second-choice drug (and the drug of choice for patients allergic to penicillin) is erythromycin. Ampicillin and amoxicillin have often been used to treat group A streptococcal infections. They are absorbed better than penicillin, however they have never been shown to have any advantages in the treatment of group A streptococcus. In addition, if the pharyngitis is caused by Epstein-Barr virus, ampicillin treatment may induce a whole-body rash.
Recurrent pharyngitis is often a challenging clinical problem. Several explanations have been proposed as to why penicillin treatment of group A streptococcal pharyngitis can fail: (1) Although group A streptococci are always penicillinsusceptible, tolerance (the bacteria are inhibited but not killed) has been suggested as one reason for recurrent infection. (2) Production of Beta-lactamase by staphylococci or by anaerobes such as Bacteroides species can inhibit the effect of penicillin in the pharynx. (3) The infection may in reality be a reinfection, e.g., from family members. (4) The peak serum levels of penicillin V vary significantly from person to person, indicating differences in the absorption of the drug. After the use of penicillin V in the first drug regimen proves ineffective, erythromycin or a second generation cephalosporin are valid choices for the second treatment. Also, dicloxacillin has been successfully used for children with recurrent streptococcal pharyngitis. Tonsillectomy is the best treatment to eliminate the series of persistent infections.
With the exception of very rare infection by certain of the other pharyngeal bacterial pathogens mentioned next (i.e.) Corynebacterium diphtheriae and Neisseria gonorrhoeae), antimicrobial therapy is of no proven benefit in the treatment of acute pharyngitis due to bacteria other than the group A streptococcus. It is therefore extremely important for physicians to be able to exclude the diagnosis of group A streptococcal pharyngitis to prevent inappropriate administration of antimicrobials to large number of patient with pharyngitis. The administration of such therapy unnecessarily exposes patients to the associated expense and hazards, and it may also contribute to the emergence of antibiotic-resistant bacteria, which is being reported with increasing frequency in the United States and elsewhere.
Neisseria gonorrhea
Neisseria are Gram-negative diplococci. Of the two pathogenic types, Neisseria gonorrhea can cause pharyngitis with exudate. Diagnosis requires a certain index of suspicion and the appropriate laboratory tests. Gonococcal pharyngitis should be suspected in those individuals with a suggestive sexual history. Gram stain revealing the presence of multiple pairs of bean-shaped, Gram-negative diplococci within a neutrophil is highly characteristic of gonorrhea.. Sensitivity of the gram smear is 95%, however, the specificity is only 50 to 70% because of the presence of other bacteria with similar morphology. Culture should always be done. N. gonorrhea grows well on chocolate agar with added carbon dioxide. Currently, rapid direct detection from pharyngeal exudate via nucleic acid probes are available and may provide results before the patient leaves the clinic. Due to the development of penicillinase-producing . N. gonorrhea, penicillin is no longer used to treat gonococcal infections. Treatment of choice is 125 mg single intramuscular dose of Ceftriaxone plus Doxycycline, 100mg PO twice daily for 7 days.
Corynebacterium diphtheriae
Corynebacterium diphtheriae, the causative organism of diphtheria, produces a powerful exotoxin that is absorbed from the site of infection on the surface of the body and carried to the heart and nervous system where it causes severe damage. Human cases or carriers are the sole source of infection, which is spread by close contact. The organism is transferred by droplets or by contaminated articles. Diphtheria is especially common in children younger than 10 years of age. Although in the past, this was a common cause of pharyngitis, today it is rarely seen thanks to routine vaccination of infants and children.
C. diphtheria is a slender, non-motile, non-spore forming Gram-positive bacillus. Laryngopharyngitis due to C. diphtheria in the inadequately immunized patient is characterized by systemic symptoms from the diphtherial toxin production that are out of proportion to the pharyngeal examination. The patient is often fatigued, lethargic, and tachycardic beyond what might be expected from the patient’s fever. Classically, there is a grayish membrane extending to the pharynx and often to the larynx rather than the tonsils. Extensive diphtheria of the throat is always accompanied by marked swelling of the neck resulting from enlargement of lymph nodes and edema of surrounding tissues.
The painless swelling feels solid, and it is difficult to palpate the underlying lymph nodes. Failure to examine the throat of a patient with ‘bull-neck’ diphtheria may lead to a mistaken and tragic diagnosis of mumps. The child with serious diphtheria looks pale, limp, and toxic, whereas the child with mumps looks comparatively well. Moreover, the swelling of mumps lies superior to that of diphtheria and usually fills the hollow behind the angle of the mandible. Careful inspection of the throat settles the diagnosis.
Definitive diagnosis depends on the isolation of C. diphtheriae from local lesions. C. diphtheriae forms gray to black colonies on selective media containing potassium tellurite. The microbiology laboratory should be notified that diphtheria is suspected so that the appropriate culture media can be used. Polymerase chain reaction should also be performed on all primary isolates to screen for toxinogenicity.
Treatment for diphtheria is based on clinical diagnosis without definitive laboratory confirmation, since each day of delay is associated with increased mortality. First, the airway is secured and the patient is volume resuscitated. After allergy to horse serum has been ruled out by history and skin testing, the patient should be given diphtheria antitoxin. Epinephrine should be readily available. Antibiotics have little effect in treating acute wounds, however erythromycin, penicillin G, rifampin, or clindamycin is recommended by most authorities for the purpose of eradicating the carrier state in patients to prevent its spread.
Prevention by vaccination has led to diphtheria becoming an extremely rare occurrence. The vaccine is trivalent including diphtheria toxoid, tetanus toxoid and pertussis (DTP) and is ideally administered at 6 weeks of age, then 2 subsequent doses after intervals of 4 to 8 weeks, and a fourth dose at 6-12 months after the third.
Treponema Pallidum
Treponema pallidum is the causative agent of syphilis, a venereal disease first recognized in the 16th century as an acute and often fatal disease. The discovery of T. pallidum in syphilitic material was made by Schaudinn and Hoffman in 1905. It is a member of the Spirochete family, which also includes other pathogenic bacteria such as Borrelia, Leptospira and Fusobacteria (present in the normal oropharyngeal flora). The morphology of spirochetes differ from that of other bacteria in that they have a flexible, peptidoglycan cell wall around which several axial fibrils are wound. These fibrils form a structure referred to as endoflagella.
T. pallidum infections are acquired from direct sexual contact with an individual who has an active primary or secondary syphilitic lesion. The spirochete reaches the subepithelial tissues through inapparent breaks in the skin or by passage between the epithelial cells of a mucous membrane. They then begin to multiply locally. A primary lesion then forms 2 to 10 days after infection as an indurated swelling at the site of infection. The surface necrosis to yield a hard-based ulcerated, nontender lesion termed, chancre. The basic pathologic lesion is an endarteritis. There are several stages of syphilis: Primary, which progresses to secondary which may either spontaneously resolve or progress to tertiary or latent syphilis. Syphilis may also be congenital.
Primary syphilis, as described above, is characterized by a single ulcer at the site of infection, which persists for approximately 3 to 8 weeks in the untreated patient, and then resolves spontaneously. Clinically it is also associated with regional painless enlargement of lymph nodes.
Diagnosis is made by dark-field or direct fluorescence antibody microscopy. Often microscopy is not available, however, and therefore serology is often used to make the diagnosis. Rapid plasma reagin (RPR) is the most frequently used initial screening tool for syphilis. It is based on detection of a nonspecific antibody called reagin produced along with specific antitreponemal antibody in response to the organism. This test is highly sensitive for primary and secondary syphilis but not for delayed disease.
The reagin titer reflects the activity of the disease. If the RPR is positive, tests specific for T. pallidum should be done which include the fluorescent treponemal antibodyabsorption (FTA-ABS) test and the microhemagglutination assay for antibodies to T. pallidum (MHA-TP). Treatment is benzathine penicillin G, 2.4 million units single dose IM (1.2 million units in each buttock). For those with penicillin allergy, tetracycline, 500 mg PO 4 times daily of doxycycline, 100 mg PO twice daily should be given for two weeks.
The disease is then silent for 2 to 10 weeks, during which a disseminated secondary stage develops. It is characterize by a symmetric mucocutaneous maculopapular rash and generalized nontender lymph node enlargement. About one third of cases develop painless mucosal warty erosions called condylomata lata. In one third of untreated cases, host immune responses appear to resolve the infection. In the remainder, the illness enters a dormant or latent state. Diagnosis and treatment is the same as in primary syphilis.
Positive serologic testing for syphilis in the absence of clinical disease is consistent with latent syphilis. There are two types of latent syphilis: Early latent and late latent. Early latent syphilis , which occurs within 2 years of infection, is potentially transmissible because relapses associated with spirochetemia are possible. Late latent syphilis, which occurs more than 2 years after infection, is associated with immunity to relapse and resistance to reinfection: About one third of cases do not progress beyond this stage.
Tertiary syphilis occurs in approximately one third of untreated patients. Clinical manifestations of tertiary syphilis present on average of 15-20 years after initial infection but may happen as early as 5 years. Sequela include the following: CNS – Tabes dorsalis – demyelination of the posterior columns resulting in ataxia, loss of sensation of position, pain and temperature, meningovascular syphilis resulting in paresis, mental status changes (decreased memory to frank psychosis), and cardiovascular syphilis resulting in the development of aneurysms of the aorta.
Fusobacterium necrophorum
Fusobacterium necrophorum is an anaerobic Gram-negative bacillus. It is important in the discussion of pharyngitis as it is the etiologic agent of the uncommon but life threatening condition of Lemierre Syndrome. This condition, previously known as post-anginal septicemia, was first described in 1936. It consists of thrombophlebitis of the internal jugular vein following an episode of acute pharyngitis or tonsillitis. Typical presentation is a patient with high fever, lateral neck pain, and swelling. Usually, these symptoms occur after the symptoms of pharyngitis subside. Depending on the degree of inflammation there may be a marked decrease in range of motion of the neck due to parapharyngeal space involvement. Untreated, it progresses to septic thromboemboli to seeding of the lungs, renal impairment, hepatitis, peritonitis and joint involvement.
Diagnosis is made by clinical factors and CT scan with contrast confirming thrombosis of the internal jugular vein. Treatment in the past consisted of ligation and resection of the internal jugular vein, however this is no longer advocated. Initial treatment should consist of drainage of any suppurative processes (peritonsillar, parapharyngeal, or retropharyngeal abscess) followed by supportive therapy in an ICU setting with intravenous antibiotics.
Other Bacterial infections
Mycoplasma pneumoniae has been isolated from patients with pharyngitis. A similar intracellular organism, Chlamydia pneumoniae, has also been linked to pharyngitis. Both of these pathogens also cause other respiratory tract infections, such as pneumonia. There is no current evidence that antimicrobial treatment will eradicate these pathogens or shorten disease. However, many physicians treat despite these facts.
Fungi
Candida albicans
Candida albicans is normally present in small numbers in the oral cavity. This colonization is aided by the ability of C. albicans to adhere to mucosal cells, a feature that distinguishes it from most other Candida species. Factors that allow C. albicans to increase its relative proportion of the flora (antibiotic therapy), that compromise the general immune capacity of the host (leukopenia or corticosteroid therapy), or that interfere with T lymphocyte function (AIDS, transplant patients taking anti T-cell medications such as cyclosporin, leukemia) are often associated with local and invasive infection. Diabetes mellitus also predisposes to C. albicans infection.
Superficial invasion of the mucous membranes by C. albicans produces a white, cheesy plaque that is loosely adherent to the mucosal surface. The lesion is usually painless, unless the plaque is torn away and the raw, weeping, invaded surface is exposed. Oral lesions called thrush, occur on the tongue, palate, and pharynx and in more severe cases may extend into the larynx and esophagus. Laryngeal candidiasis is not uncommon in patients who overuse inhaled steroids for the treatment of asthma or chronic obstructive pulmonary disease.
Diagnosis is usually made clinically in a patient who is immunosuppressed from one of the above mentioned conditions. Exudate or epithelial scrapings examined by KOH preparation or Gram smear can be done and demonstrate abundant budding yeast cells; if associated hyphae are present, the infection is almost certainly caused by C. albicans.
Treatment for oropharyngeal candidiasis is nystatin suspension taken as 10 –15 milliliters mouth rinses five times per day. More severe or refractory infections may require oral fluconazole 400 mg PO bid. Disseminated candidiasis is treated with amphotericin B.
Other Mycoses
Many other fungi can affect the head and neck, however, specific infection of the pharynx is extremely rare. These include Cryptococcus neoformans, Rhinosporidiosis seeberi, Histoplasma capsulatum, Blastomyces dermatitidis, and Paracoccidioidomycosis brasiliensis.
Granulomatous Disease of the Pharynx
A microscopic aggregation of epithelioid cells, usually surrounded by a collar of lymphocytes, is referred to as a granuloma. This pattern of inflammation that is characteristic of type IV (cell–mediated) hypersensitivity reaction is called granulomatous inflammation. There are a large number of disease processes that result in granuloma formation ranging from infectious processes such as tuberculosis and other mycobacteria, leprosy, and parasites, to systemic diseases such as Wegener’s granulomatosis, sarcoid, and Crohn’s disease.
Wegener’s Granulomatosis
Wegener’s granulomatosis (WG) is a distinct clinicopathologic entity characterized by granulomatous vasculitis of the upper and lower respiratory tracts together with glomerulonephritis. In addition, variable degrees of disseminated vasculitis involving both small arteries and veins may occur.
The histopathologic hallmarks of WG are necrotizing vasculitis of small arteries and veins together with granuloma formation. Immunopathogenesis of this disease is unclear, although the involvement of upper airway and lungs suggest an aberrant hypersensitivity response to an exogenous or even endogenous antigen that enters through or resides in the upper airway.
A typical patient presents with severe upper respiratory tract finds such as paranasal sinus pain and drainage and purulent or bloody nasal discharge with or without nasal mucosal ulceration. Nasal septal perforation may follow, leading to saddle nose deformity. WG usually does not affect the pharynx directly but inflammation in this area is usually secondary to irritation from post-nasal drip. More commonly, WG may involve the larynx. The subglottis is the most common single site of involvement, however granulomas can usually be found throughout the larynx.
The diagnosis of WG is a clinicopathologic one made by the demonstration of necrotizing granulomatous vasculitis on biopsy of appropriate tissue in a patient with clinical findings of upper and lower respiratory tract disease together with evidence of glomerulonephritis. Pulmonary tissue preferably obtained by open thoracotomy, offers the highest diagnostic yield. Nasal biopsy may also be performed if involvement is evident.
Although specific variations in treatment exist, the treatment of choice in this disease is cyclophosphamide given in doses of 2 mg/kg per day orally. This should be continued for 1 year following the induction of complete remission and gradually tapered and discontinued thereafter. At initiation of therapy, glucocorticoids should be administered together with cyclophosphamide. Specifically prednisone 1 mg/kg per day should be given with gradual conversion to alternate-day schedule followed by tapering and discontinuation after approximately 6 months. Using the above regimen, the prognosis of this disease is excellent; marked improvement is seen in more than 90% of patients and complete remission is seen in 75% of patients.
Tuberculosis
Mycobacterium tuberculosis is a Gram-negative bacillus that demonstrates the staining characteristic of acid-fastness. Tuberculosis is a disease of great antiquity that reached epidemic proportions during the major periods of urbanization in the 18th and 19th centuries. A resurgence of the disease has been seen in the past several decades in conjunction with the emergence and rising prevalence of HIV and AIDS.
Involvement of M. tuberculosis in the head and neck is uncommon. Specific localization to the pharynx is even less common. However, mycobacterial pharyngotonsillitis does occur and results from expectoration of infected sputum from pulmonary involvement. Clinical exam reveals an erythematous, infiltrative, granular or ulcerated surface mucosa. Tuberculous laryngitis is the most common granulomatous disease of the larynx and most often involves the posterior third of the larynx.
Diagnosis is made by demonstrating the tubercle bacilli in the sputum, urine, body fluids, or tissues of the patient. The staining characteristics of M. tuberculosis allow its ready identification. Sputum culture adds to the diagnostic yield and also permits the specific identification of acid-fast bacilli and the determination of drug susceptibility. Isolation from clinical specimens usually requires 4 to 8 weeks.
Daily treatment with regimes including isoniazid and rifampin for 9 to 12 months represents the most effective treatment available and is capable of achieving a favorable outcome in 99% of patients. May clinicians add a third drug initially until the results of sensitivity tests become available; pyrazinamide is the optimal third drug, and ethambutol is also effective.
Sarcoidosis
Sarcoidosis is a chronic, multisystem disorder of unknown cause characterized in affected organs by an accumulation of T lymphocytes and mononuclear phagocytes, noncaseating epithelioid granulomas, and derangements of the normal tissue architecture.
Sarcoidosis is a relatively common disease affecting individuals of both sexes and almost all ages, races, and geographic locations. Females appear to be slightly more susceptible than males. Most patients are between the ages of 20 and 40 years.
Clinical manifestations may be generalized or focused to one or more organs.
The lung is almost always involved and therefore the majority of patients present with respiratory complaints. 90% of patients have an abnormal chest X-ray which usually shows hilar adenopathy. The nasal mucosa is involved in up to 20% of patients, usually presenting with nasal congestion. Any of the structures of the mouth, pharynx and larynx can be involved, particularly the tonsils. The usual finding is erythema of the tonsil or hypertrophy. Sarcoidosis involves the larynx in about 5% of cases. The epiglottis is the most common site of involvement.
Diagnosis can usually be made on clinical an radiographic findings alone, however if upper airway findings are present, biopsy of affected sites should be done to rule out other pathology and to confirm the presence of noncaseating granulomas.
Biopsy of the lung is the most common method for confirming the diagnosis, however issue samples taken from the skin, conjunctive, or lip may also be done.
Treatment of choice for sarcoidosis is glucocorticoids. Deciding when to treat is he major clinical dilemma. Because the disease clears spontaneously in about 50% of atients, and because the permanent organ derangements often do not improve with lucocorticoids, there is controversy among clinicians as to the criteria for treatment. ome us criteria based on gallium scan and level of angiotensin converting enzyme to ndicate disease activity. Others base their decision for treatment solely on severity of pecific clinical manifestations. The usual therapy is prednisone, 1 mg/kg, for 4 to 6 weeks, followed by a slow taper over 2 to 3 months. This is repeated if the disease again becomes active.
Crohn’s Disease
Crohn’s is an inflammatory disease of the bowel for which the exact etiology is unknown. It is more common in whites, occurs with an increased frequency in Jews, and exhibits some familial clustering suggesting that there may be a genetic predisposition to the development of disease.
Pathologically, Crohn’s is characterized by chronic inflammation extending through all layers of the digestive tract wall. Unlike its counterpart, ulcerative colitis, it may affect any area of the digestive tract from the mouth to the anus. Crohn’s is often discontinuous characterized by “skin lesions”. Microscopically, granulomas are most helpful in distinguishing it from other forms of inflammatory bowel disease.
The major clinical features of Crohn’s disease are fever, abdominal pain, diarrhea often without blood, and generalized fatigability. Pharyngeal involvement may be seen in 9% of patients during the course of their disease and usually follows intestinal manifestations.
RECURRENT RESPIRATORY PAPILLOMATOSIS
Introduction
Recurrent respiratory papillomatosis (RRP) is a disease of viral etiology caused by HPV types 6 and 11 which results in exophytic lesions in the airway. There are two distinct forms recognized: juvenile onset (most aggressive) and adult onset (less aggressive). RRP is the most common benign neoplasm of the larynx in children and the second most cause of pediatric hoarseness. Juvenile RRP, the main focus of this discussion, is mostly diagnosed between the ages of 2 and 4 yrs old with a delay of symptom onset at approximately 1 year (Silverberg). Children with RRP are diagnosed before their 5th birthday 75% of the time.
Incidence is estimated at 2 per 100,000 in adults and 4 per 100,000 in children which equals over 15,000 surgical procedures totaling in excess of one-hundred million dollars per year for childhood procedures! (Derkay, 1995) Plainly stated, 1,500-2,000 new cases of childhood onset RRP occur in the US each year.
RRP not only involves the larynx but can spread into the entire aerodigestive tract. Children whose RRP was diagnosed at ages < 3 years old have 3.6 times more chance to have > 4 surgeries per year and almost twice as likely to have 2 or > anatomic sites affected than children diagnosed at > 3 years old. (Wiatrak) .
Although RRP is a benign disease it ahs extremely morbid potential due to its airway involvement and potential for malignant transformation.
Etiology
HPV is the etiological agent in RRP, first discovered in laryngeal papillomas in 1982. HPV is also the cause of the 2nd most common female cancer—cervical cancer. It is also associated with anogenital cancers such as anal, penile, vulval, and vaginal carcinomas. (Goon) HPV is a DNA containing, nonenveloped, icosehedral (20 sided) capsid virus with double stranded DNA ~ 7,900 bp long. HPV types 6 and 11, as stated earlier are the most prevalent types with type 11 having the worst prognosis. Other types of HPV encountered in RRP include types 16 and 18 (highly malignant) and types 31 and 33 (malignant potential more than types 6 and 11, but less than types 16 and 18). It is believe that HPV infects cells by getting set up in the basal layer and releasing its DNA into the cell. This DNA is then transcribed into RNA which is then translated in to viral proteins. These proteins will go on to assemble new virions and once they are released, the cycle will continue as these virions infect other cells. The HPV genome is made up of early genes (designated “E”) and late genes (designated “L”) each with unique purposes. Specifically, HPV L1 and L2 genes encode viral capsid proteins while E1 and E2 are responsible for replication and transcription. E4 aids in virus release from infected cells and E6/E7 have transforming abilities. (Goon) Interestingly, following infection of stem cells in the basal layer, the infection can be actively expressed or become latent. To demonstrate this fact, HPV DNA can be detected in normal appearing mucosa in patients with RRP who are in remission. The fact that infection can be latent explains why reactivation and clinical occurrence can take place following years of remission. Host immune response is thought to play a role in the pathogenesis of HPV lesions. Malfunction of cell mediated immune response in patients with juvenile-onset RRP has been demonstrated. Some papillomas may evade immune surveillance and permit a more rapid progression of the disease.
Below is a graphic from Nature Reviews to help illustrate the process of HPV infection as briefly described above:
Epidemiology
Childhood-onset RRP is most often diagnosed between the ages of 2 and 4 with an estimated incidence of 4 per 100,000 (2 per 100,000 in adults). Briefly, adult RRP peaks between ages of 20-40 yrs and has a slight male predilection. Adult-onset RRP could reflect activation of a virus present at birth or infection acquired in adolescence or adult life. A U.S. survey of practicing otolaryngologists revealed that half the adults with RRP required fewer than 5 procedures over their lifetime as opposed to <25% of children who can share this same statistic. In the U.S., the mean number of surgical procedures per child is estimated at 19.7 with an average of 4.4 procedures per year. (Derkay, 1995)
In terms of distal airway spread and malignant transformation, HPV 11 is more likely to be the culprit than HPV 6. HPV 11 is thought to integrate itself into the host genome and mutate tumor suppressor p53. (Rady).
Transmission
The exact mode of HPV transmission remains unclear but the most likely method of maternal-fetal HPV transmission is through direct contact via the birth canal. This may explain the clinical observation that most children in whom recurrent respiratory papillomas develop are delivered vaginally to mothers with a history of condylomata. Statistics reveal that not only are children with RRP commonly born vaginally to mothers with a history of condylomata, but they are first-born as well. This fact is significant because primigravid mothers have a large 2nd stage of labor and prolonged infant exposure to the virus leads to higher risk of infection. Maternal condylomata during pregnancy was associated with a 200-fold increase in risk of RRP in the child. (Silverberg) In one group of children with juvenile laryngeal papillomatosis, 54% had a maternal history of vulval condyloma at delivery (Hallden) Despite the apparent close association between maternal condylomata and RRP development, few children exposed to genital warts at birth actually develop clinical symptoms. The estimated risk of a child contracting the disease from a mother who has an active condylomatous lesion and delivers vaginally is 1 in 400. What separates this one child from the other 399 is unknown at this point. Although HPV could be recovered in approximately 30% of infants exposed to HPV in the birth canal, only a small fraction of these will exhibit evidence of RRP. Another study found that in 77 mothers with condylomata at delivery, 9 children (11.6%) were later diagnosed with juvenile RRP. (Kjer) It is clear that other factors must be important in determining the development of RRP (i.e. patient immunity, timing, length and volume of virus exposure and local trauma). In contrast, to studies that link childhood RRP to mothers with genital HPV infections, adult-onset RRP may be linked to oral-genital contact. A case control study revealed that adult-onset RRP patients were more likely to have had increased sexual partners and oral sex than their controls. (Kashima)
Since the main theory regarding transmission of RRP in juvenile-onset cases is contact via birth canal, it seems reasonable that cesarean section would eradicate this risk altogether. Unfortunately, there is insufficient evidence to support cesarean section in the prevention of childhood RRP in lieu of the maternal risks associated with this procedure.
Histology and Lesion Characteristics
RRP lesions occur where ciliary and squamous epithelium are juxtaposed. Ciliated epithelium undergoes squamous metaplasia when exposed to repeated trauma and is replaced with nonciliated epithelium that creates an iatrogenic squamociliary junction. This is probably whey RRP is exacerbated in the presence of reflux. In tracheotomized patients, RRP is often found at the stoma and in midthoracic trachea (i.e. iatrogenic squamociliary junction). Histologically, the lesions are pedunculated masses with finger-like projection of nonkeratinized stratified squamous epithelium supported by a core of highly vascularized connective tissue stroma. Grossly, papillomas may be sessile or pedunculated and usually occur in exophytic clusters. They appear pinkish/white in color.
Clinical Characteristics
RRP has a hallmark triad of progressive hoarseness, stridor, and respiratory distress. Due to the fact that the most common symptoms are related to airway obstruction, many children are misdiagnosed with croup, asthma, or chronic bronchitis. Any child with voice changes, obstructive airway symptoms should get fiberoptic laryngoscopy or direct laryngoscopy to rule out neoplasia with RRP being high on the differential. RRP presenting in the neonatal period is thought to be a negative prognostic factor with a greater likelihood for mortality and need for tracheotomy.
Extralaryngeal spread of respiratory papillomatosis has been identified in 13-30% of children and in 16% of adults with RRP. The most common sites for RRP are as follows:
- Limen vestibuli,
- Nasapharyngeal surface of soft palate
- Midline of laryngeal surface of epiglottis
- Upper and lower margins of ventricle
- Undersurface of vocal folds
- Carina
- Bronchial spurs
The most frequent extralaryngeal sites were (in order of frequency): oral cavity, trachea, and bronchi.
There is controversy as to whether tracheostomy exacerbates distal spread of disease. Certain authors such as Cole et al reported that increased tracheal spread occurred in their patients with RRP who received tracheotomy. (Holland) However, in opposition to this , Shapiro et al did not believe that placement of tracheotomy contributed to distal spread of disease.
Patient Assessment/Gathering history
Hoarseness, although a common/benign complaint in children, always indicates some abnormality of structure or function. Important information to gather is:
- Time of symptom onset
- Airway trauma? Previous intubation?
- Characteristics of cry
Voice assessment should involve determination of the quality of voice:
- Low pitched, coarse, fluttering voice suggests subglottic lesion
- High pitched cracking voice aphonia or breathy voice suggests glottic lesion or subglottic lesion
Other important things to assess:
- Age of onset
- Rate of progression
- Associated infection
- History of trauma or surgery
- Presence of respiratory or cardiac distress
Also, it is important to not be fooled by stridor that is present at birth. Although this is most likely laryngomalacia, subglottic stenosis, vocal cord paralysis or vascular ring, you must remember that neonates also can present with papillomatosis. In addition, it is important to review the perinatal period for a history of maternal or paternal condylomata.
Physical exam:
- Assess respiratory rate and degree of distress first
- Observe for flaring of nasal ala, use of accessory neck/chest muscles
- Cyanosis or air hunger( child my sit with neck hyperextended)
- Be careful in very sick children. Detailed physical exam should be done in the OR, ER, or ICU where equipment for resuscitation, intubation, and tracheotomy can be performed
Malignant Transformation
Malignant transformation of laryngeal or pulmonary lesions is considered rare and is estimated to occur in 1-7% of patients with RRP (Gerien). Typically, malignant transformation occurs in those patients with advanced disease, usually pulmonary extension of RRP, who are subsequently diagnosed with lung carcinoma. This lung carcinoma will have the same virus as benign papillomas in the larynx. This usually occurs during the third or fourth decade of life. As described earlier, the majority of these lesions contain HPV type 11 as opposed to type 6. The reliability of prognostic factors other than age of onset for predicting severity of RRP (including malignant transformation) is undefined. Gerien et al demonstrated that the average duration of RRP until malignant transformation lies within a range of approximately 19-35 yrs and approximately 9-21 yrs from the time pulmonary extension is diagnosed.
Treatments
Surgical debridement is the mainstay of treatment with the aims of maintaining a safe airway and a good voice. The most favored method currently is usage of the powered microdebrider instead of CO2 laser due to decreased surgical time, less cost and better voice quality, with no increase in pain. (Pasquale)
Existing adjuvant therapy is considered “off-label” usage; however the following medical treatments have been explored thus far:
- Interferon- Has antiviral, antiproliferative and immunomodulatory effects by binding to membrane receptors and altering cell metabolism. Exact mechanism is unknown. The conclusion is that interferon is neither curative alone nor of any value as adjunctive treatment (Healy)
- Cidofovir- A cytosine analogue that is active against many DNA viruses. Precise mechanism unknown but thought to induce apoptosis and augment the immune response. Prospective studies have shown intralesional cidofovir to promote regression (partial and complete) of papillomas and decrease frequency of surgical debulking. (Tasca)
- Antivirals- Namely Ribavirin and acyclovir. Evidence proving efficacy for these is weak/anecdotal. Effect is not directed at HPV but against co-infecting viruses.
- Indole-3-carbinol- Derived from cruciferous vegetables. Thought to affect papillomas via effect on estrogen metabolism. Prospective study has shown some benefit but evidence of clinical efficacy is equivocal.
- Mumps vaccine- Unclear mechanism but authors feel that intralesional mumps vaccine promotes induction of remission in children with RRP.
- Control of GERD- LPR is thought to exacerbate severity of RRP. Prospective study by authors like McKenna Demonstrates improved control of RRP and ometimes remission when GERD is controlled. (McKenna)
- HspE7- Recombinant fusion protein of Hsp65 of M. bovis and E7 protein from HPV 16. Open label study has shown statistically significant decrease in the patient’s annual frequency of surgery and decreased absolute number of surgeries. (Derkay, 2005).
Conclusion
RRP although rare, is a debilitating disease with grave consequences to the airway if left unnoticed and uncontrolled. Currently great locoregional control is possible through frequent surgical debridement which adjuvant treatment may be able to decrease. At this point, not only must more research be undertaken to find a solution, more research must be performed to understand more about the problem—the HPV virus. Fortunately, with the advancements currently accomplished, the future looks promising.
SURGICAL MANAGEMENT OF OBSTRUCTIVE SLEEP APNEA IN ADULTS
Background Information
Sleep apnea exists among a spectrum of disorders known as sleep disordered breathing. These range from primary snoring to obesity hypoventilation syndrome. Primary snoring is characterized by a snoring with a lack of nighttime awakenings and daytime sleepiness. This is followed by upper airway resistance syndrome (UARS), which has frequent nighttime awakenings and daytime sleepiness, but an absence of apneic episodes. Obstructive sleep apnea syndrome (OSAS) has nocturnal episodes of apnea and oxygen desaturation that cause frequent nighttime awakenings and daytime sleepiness. Finally, patients with obesity hypoventilation syndrome, also know as Pickwickian syndrome, are obese, have daytime hypercapnea, and have some form of sleep disordered breathing.
Some facts about OSA are that it affects approximately 18 million Americans, with up to 70% of the cases associated with obesity. There is an increased incidence with age, and patients have an increased overall mortality compared to the general population. It is estimated that 38,000 deaths attributed to cardiovascular disease in the United States per year are related to OSA. Additionally, patients with OSA have a risk for motor vehicle accidents up to seven times the general population.
History
Important points of the history when interviewing a patient with suspected OSA include: the presence of daytime sleepiness and restless sleep, the use of alcohol and sedatives, mouth breathing during sleep and throughout the day, and morning headaches (especially in females). It is helpful to ask the patient about their bedtimes, awakening times, body position during sleep, caffeine intake, and menopausal status in females (postmenopausal is a risk factor for OSA). It is crucial to obtain input from the patient’s bed partner or a family member, as these patients often unaware of their symptoms. This is in part due to a lack of awareness during sleep, but can also be attributed to the gradual onset of symptoms.
Physical Exam
On physical exam, it is first necessary to note the patient’s general body habitus, describing if they are obese and whether their weight distribution is central or peripheral. The physician should look for signs of chest wall deformity and note if the patient has a systemic disorder such as achondroplasia or Marfan syndrome (both have a high prevalence of OSA). Additionally, the presence of retrognathia or micrognathia should be noted, along with a quantitative measurement of the patient’s neck size. Next, signs of nasal obstruction should be sought out, looking for a deviated septum, polyps, turbinate hypertrophy, or nasal valve collapse. On oropharyngeal exam, the size of the palate, tongue, uvula, and tonsils should be described. In OSA, patients often have an elongated palate and uvula with mucosal folding. Finally, the posterior pharyngeal wall should be examined for the presence of banding. Following the oropharyngeal exam, a thorough examination for enlarged lymph nodes, as well as an enlarged or irregular thyroid is important. Often tumors of the head and neck can present with new onset OSA.
In addition to the head and neck exam, a cardiovascular exam is important, as many OSA patients have comorbidities involving this organ system. A final important part of the physical exam is examination via flexible nasopharyngeal scope. This can further evaluate the nasal cavity for polyps and tumors, as well as for the presence of enlarged adenoids. Flexible scope will allow the physician to better examine the base of tongue and epiglottis. A thickened, omega shaped epiglottis can sometimes be found in patients with OSA.
Finally, the physician should have the patient perform the Müller maneuver. This is accomplished by asking the patient to close their mouth, hold their nose shut, and inspire with maximal effort. The physician should look for signs of airway collapse at the palate, base of tongue, and lateral pharyngeal walls. The collapsibility of each structure can be quantified on a scale of 0 to 4, with 0 indicating minimal collapse, and 4 indicating complete collapse.
Diagnostic Modalities
After history and physical exam, there are three main methods of diagnosis for OSA. These include questionnaires, cephalometric analysis, and polysomnography.
A variety of questionnaires exist to aid in the diagnosis of OSA. The first type measures the degree of sleepiness throughout the day. This type includes the Epworth and Stanford sleepiness scales. The Epworth sleepiness scale is probably the most widely used questionnaire, and asks the patient to rate their sleepiness during various everyday activities such as reading, watching TV, and driving. The Stanford sleepiness scale is similar, but asks the patient to rate their sleepiness at each hour of the day. Other types of questionnaires involve quality of life measures that are specific to OSA. Downsides of these questionnaires are that they are not highly specific to OSA, with diseases such as narcolepsy and restless leg syndrome often having positive questionnaires.
 The next type of diagnostic modality available is called cephalometric analysis. This involves take lateral plain radiographs of the face and skull base. Several points are plotted in order to evaluate the position of the mandible in reference to the skull. Using these points, several linear and angular measurements are taken. Important for the diagnosis of OSA is the size of the posterior airway space, the length of the soft palate, and the distance from the mandible to the hyoid bone. These measurements are especially beneficial for decisions concerning surgical management.
The next type of diagnostic modality available is called cephalometric analysis. This involves take lateral plain radiographs of the face and skull base. Several points are plotted in order to evaluate the position of the mandible in reference to the skull. Using these points, several linear and angular measurements are taken. Important for the diagnosis of OSA is the size of the posterior airway space, the length of the soft palate, and the distance from the mandible to the hyoid bone. These measurements are especially beneficial for decisions concerning surgical management.
The gold standard for the diagnosis of OSA is the sleep study, or polysomnography. Although alternatives exist, the ideal sleep study takes place overnight at a sleep lab. A variety of measurements are taken during the study, including: pulse oximetry, electroencephalogram (EEG), electrooculogram (EOG), electrocardiogram (ECG) electromyogram (EMG), degree of respiratory effort, amount of oral and nasal airflow, and limb and body movements. Terms that are important for the diagnosis of sleep apnea are apnea, hypopnea, and apnea/hypopnea index (AHI). Apnea is defined as a lack of ventilation for greater than or equal to 10 seconds with signs of arousal. Hypopnea is defined as a decrease in respiratory movement with a drop in oxygen saturation or with signs of arousal. Finally, the AHI, which is also known as the respiratory disturbance index (RDI) is defined as the number of apneas plus the number of hypopneas divided by the number of hours of total sleep. While there is a large amount of data generated during sleep studies, often the diagnosis of OSA is made only by the RDI. However, other important parameters for diagnosis and determination of severity are: lowest oxygen saturation, number of desaturation episodes below 90%, and the total amount of time below 90% oxygen saturation.
Going back to the spectrum of sleep disordered breathing, there are more technical terms based on polysomnography for each of the disorders. Primary snoring has an RDI below 5. UARS has an RDI below 5, but an arousal index above 5 (number of arousals per hour as determined by EEG). OSA must have an RDI above 5 and oxygen desaturation episodes below 90%. For Pickwickian syndrome, the patient must have a BMI greater than 30 kg/m2, daytime hypercapnea with a PaCO2 of at least 45 mmHg, and some type of sleep disordered breathing.
Surgical Indications
Surgical indications for the management of OSA include an RDI above 15, an RDI above 5 with daytime sleepiness, oxygen desaturation episodes below 90%, or the presence of cardiac arrhythmias. Underlying all of these criteria is that there must have been an unsuccessful trial of medical therapy, or CPAP. When patients are completely compliant with CPAP, it is nearly a universal cure. However, 100% compliance is difficult to maintain, and patients often seek surgical alternatives with the goal of becoming free of the CPAP machine or to help them better tolerate the machine. Finally, as these patients often have many medical comorbidities, they must be considered medically stable for surgery.
Surgical Management
Surgical management of OSA includes a wide variety of procedures that vary in their invasiveness and success rates. Most of the studies that have examined the success rates are retrospective chart reviews and vary greatly in their reported numbers. Generally, success is defined as a drop in the RDI by either 50% or 20 total points.
The first types of procedures done for OSA are rhinological. Increased nasal resistance may increase negative pressure in the airway during inspiration, causing airway collapse. The main procedures used to correct nasal obstruction are septoplasty, turbinate reduction, and functional endoscopic sinus surgery. These serve to correct problems such as a deviated septum, allergic rhinitis, nasal polyposis, and chronic rhinosinusitis. These are usually considered adjuncts to other procedures or treatments, with the goal often being improvement of nasal CPAP compliance. The clinical usefulness of these procedures for OSA is considered controversial, as some studies have shown that septoplasty can increase the severity of OSA.
The next type of procedure involves palatal reduction. This is typically an uvulopharyngopalatoplasty (UPPP), which is the most common procedure performed for OSA. The UPPP trims excess palatal length along with the uvula, and is often combined with a tonsillectomy. Success rates are reported to be between 40 and 50%. However, these figures drop to 6% if macroglossia is present. This procedure, like many of the other procedures for OSA, has a low complication rate, with most problems arising from airway compromise.
More aggressive and less commonly performed procedures involve the tongue base. The first type is the tongue base suspension, where a permanent suture is run through the base of tongue and attached to a screw placed on the inner aspect of the anterior mandible. This serves to prevent the tongue from collapsing posteriorly during sleep. An advantage of the procedure is that it is quick, with operation times reported as only 20 minutes. This procedure is considered to have a variable success rate of between 20 and 82%. A newer type of tongue base procedure is the tongue base reduction. This is usually an office based procedure performed with the Coblator. It can be done multiple times to achieve the desired result. The success rates reported in the literature are more promising at between 60 and 85%..
The next procedure is the genioglossus advancement. This is accomplished by performing an osteotomy of the anterior mandible with advancement of the bone segment one width of the mandible. The segment is then rotated to prevent retraction, and secured in place with a titanium plate or screw. The procedure serves to reduce tongue collapse in a similar manner to the tongue suspension. The success rate has been reported to be anywhere from 23 to 77%. Associated complications are injury to the genioglossus muscle and mental nerve.
A more aggressive procedure for OSA is the hyoid suspension. In this procedure an external incision is made on the neck, and the hyoid is dissected inferiorly and advanced over the thyroid cartilage. The hyoid is held in place by placing a permanent suture. This procedure is typically performed in conjunction with a genioglossus advancement or UPPP. As with many of the other procedures, it has a variable success rate of between 17 and 65%. In addition to the high failure rate, another disadvantage of the hyoid suspension is that it often causes dysphagia.
The next procedure is the maxillomandibular advancement. For this procedure, a Lefort I osteotomy is created in the maxilla, along with bilateral ramus osteotomies and an anterior inferior mandibular osteotomy. Each segment is advanced 10-14 mm while ensuring that proper dental occlusion is maintained. This serves to enlarge the posterior airway and is the only procedure mentioned thus far with a reproducible high success rate (75-100%). One downside of the procedure is that it significantly alters the patient’s facial appearance. However, reports have stated that patients are usually happy with the change.
The final procedure is the tracheotomy. This is only indicated for the presence of severe, life-threatening OSA. It is the only procedure that has consistently shown 100% success rates for severe OSA. However, even this procedure is not completely curative for patients that already have cardiopulmonary decompensation. Many patients with Pickwickian syndrome fall into this category and have to go on the ventilator during sleep. Due to obvious quality of life and social stigma issues, the tracheotomy is rarely done for treatment of OSA.
Surgical Planning
With several different procedures available to help patients with OSA, it can be difficult to decide which is best suited for each individual patient. To make this decision, determination of the site or sites of obstruction, as well as the severity of OSA must be made. The usual levels of obstruction are: the nasal cavity/nasopharynx, the palate/oropharynx, and the base of tongue/hypopharynx. This information can be obtained via physical exam, with the Müller maneuver being particularly helpful, and cephalometric analysis.
Next, the severity of the patient’s OSA should be determined by polysomnography. Patients with mild OSA have an RDI of less than 20 and a lowest saturation of oxygen greater than 85%. Patients with moderate OSA have an RDI between 20 and 40 and a lowest saturation of oxygen of greater than 80%. Patients with moderate/severe OSA have an RDI between 40 and 60 and a lowest saturation of oxygen of greater than 70%.
Finally, patients with severe OSA have an RDI less than 60 and a lowest saturation of oxygen less than 70. Generally, as the severity of OSA increases, so should the invasiveness or aggressiveness of the procedure. The surgical planning should also keep in mind the patient’s overall desires, preferences, and goals. Finally, the patient’s health status and ability to tolerate each type of procedure should be considered. Overall, the goal is to minimize the surgical intervention and avoid unnecessary surgery, while helping the patient achieve their goal for the treatment.
Protocol for Surgical Management
A specific protocol was developed at the Stanford Sleep Center for the surgical planning of OSA. The protocol involves having a presurgical evaluation with physical exam including flexible scope examination, cephalometric analysis, and a sleep study. Then, the patient undergoes a procedure or combination of procedures based on the site of obstruction and severity of disease. A sleep study is completed 6 months postoperatively. If the surgery is not found to be a success, the patient receives a maxillomandibular advancement.
Based on this protocol, a prospective study was completed that had 135 patients with mild to moderate OSA, and 42 patients with severe OSA. Their goal was to minimize surgical interventions and avoid unnecessary surgery while achieving a cure. Success in the study was defined as an RDI reduction of at least 50% or an absolute drop of at least 20. The study was completed in two phases. In phase I, all patients were placed into one of three groups. Group 1 was determined to have obstruction at the level of the oropharynx and received a UPPP. Group 2 had combined obstruction at the oropharynx and hypopharynx and received a genioglossus advancement, a hyoid suspension, and a UPPP. Group 3 only had obstruction at the hypopharynx and received a genioglossus advancement and a hyoid suspension. Of note is that a hyoid suspension was not performed if intraoperatively the patient was considered to have achieved adequate enlargement of the hypopharynx with genioglossus advancement alone, or if airway edema was considered to be likely after the genioglossus advancement was completed. Patients who did not have success in phase I as determined by a 6 month postoperative sleep study moved onto phase II, which was maxillomandibular advancement. The success rates for phase I were 71-78% for mild to moderate OSA and 42% for severe OSA. For phase II of the study, the success rate for patients that failed phase I was 100%. As evidenced by the high success rate of the study, the Stanford protocol was recommended as a method of achieving a cure for OSA via surgical intervention.
Conclusion
There is a large amount of literature concerning the surgical treatment of OSA. Many of the procedures have variable success rates that may make physicians wary of performing them as a method of treatment. In light of this, more prospective studies are needed that specifically compare these procedures alone or in combination. Additionally, the studies should be based on procedures that are specifically tailored to the sites of airway obstruction in the patient.
Also of note is that the accepted definition of success is often not acceptable clinically. If a patient has an RDI of 35 and it drops to 15 with intervention, this is considered a success. However, the patient still has an increased overall mortality based on their RDI. Additionally, the RDI does not correlate completely with the amount of daytime sleepiness or decrease quality of life they are experiencing. Quantitative measures that have been found to better correlate with these symptoms are total oxygen desaturation time, number of desaturation episodes below 90%, and the lowest oxygen saturation value.
Finally, in research studies and in clinical practice, patients often feel subjectively better after surgical intervention and are reluctant to undergo further studies. It should be emphasized to patients that postoperative sleep studies are always important to access the effect of their procedure. Overall, OSA is an underdiagnosed and undertreated disease that affects a large portion of the population. As the population becomes more obese, so will the number of people with OSA, thus making it imperative that further research be conducted to discover practical and effective therapy.
DISCUSSION: Vicente Resto, MD, PhD, FACS
Andrew, that was a very nice review of a topic that’s becoming increasingly relevant and recognized. I have several comments on it: first of all we can never forget the fact that CPAP cures all apnea. It really does. There are issues with treatment with CPAP as whether you are going to have a patient who is going to be compliant. Compliance has been studied some and to a degree this is correlated with the amount of pressure required to overcome the collapse and in others it’s just behavioral. For example, some people travel a lot and others simply don’t want to be bothered with a hose and a mask every night. The numbers out there are about 50% for compliance. In my personal practice I tailor my surgery around the patient’s disposition towards CPAP.
On another separate note, it’s clearly important to have a gradation of surgical results so you can have a method if following just how much of a contribution the surgical procedure(s) have made to the patient’s health and comfort. The Stanford group is probably the most experienced group in dealing with sleep apnea in the country. I will argue, however that from a clinically relevant perspective, reducing the RDI to less than fifty percent of their pre-intervention values is not necessarily correlative with clinical results. You can have someone with an RDI of 35 and you reduce it to 15, and they may still be somnolent enough that they may be at high risk for injury at work or while driving. Again, it’s important to understand that in order to gauge what benefits particular interventions can bring about it is one thing to think about research and clinical relevance.
In my practice I consider anybody who has an RDI greater than 10 to have an abnormal RDI. And it’s unusual that I see anybody show up in the office without symptoms having a value lower than that. So at that point in time, anything with a value greater than 10 calls for an intervention, at least the most behavioral based intervention such as weight loss or potential treatment of nasal allergy to improve patency of nasal airway at night that can perhaps improve their quality of life.
As you have pointed out, the RDI is kind of a blunt instrument, very objective and quantitative but at the same time blunt in describing disease. In my actual practice I talk to patients about CPAP as well as severity of disease. In my mind there is really two classes: mild-to-moderate, and severe. Mild-to-moderate is anywhere between 10 to 30. Greater than 30 I call severe. It matters greatly that people with RDI’s between 10 and 30 can be treated with anything. You can treat them with CPAP and they’ll get better. You can have someone who says “I don’t want to use CPAP- I’ve tried it and I hate it…” and you can offer them a number of relatively benign, well-tolerated surgical interventions and more often than not cure them. That degree of and percentage of success rate is inversely correlated with that number. The closer you get to 30 The less likely you’re going to get a complete cure.
The surgeries I’m referring to are low-complexity surgeries: nasal altering surgeries- intranasal- septoplasties, turbinate reduction, UPPP’s, tonsillectomies, which in my mind their contribution aside from tissue bulk reduction is really a secondary contribution and that is that when you do your tonsillectomy and you do your “triple P” there’s often a remodeling that occurs which tends to stiffen up the tissue increasing the benefit of the procedure.
I actually draw the line in terms of low complexity surgery there because I think the next step, talking about tongue-reposition maneuvers whether they be tongue base reduction maneuvers or genioglossal advancement procedures, both have significant potential morbidity. Whereas the previous procedures in the hands of well-trained individuals are rather well-tolerated with well-described complications that are relatively well-mannered. That changes when you transcend into the next level. As such I generally consider them as high-complexity surgeries together with mandibular/maxillary advancement, and there are things that I won’t offer a patient generally until they have a true, sincere trial of CPAP even if they come back and complain of their inability to tolerate it I will still offer them low-complexity surgery with the aim not to do away with CPAP compliance completely, but to improve their compliance by potentially decreasing pressures and improving anatomy so that they are better supported. Only at that time, in a patient with severe apnea, who has failed to tolerate CPAP, who has failed to benefit from low-complexity surgery for improved compliance, who still comes back and still has symptoms and potential co-morbidities that I will engage in high-complexity surgical procedures. I shy away from tongue-base procedures because of the potential to damage nerves such as the hypoglossal nerve which is a rather severe consequence of the this. I like to stay away from hyoid suspension, which is simply a maneuver that in my mind makes no sense in terms of increasing airway patency. In fact, we often use it when we do partial laryngeal surgery to tuck up a larynx underneath a tongue base so that you minimize aspiration. So it’s really a maneuver that works counter to what you’re looking for in sleep apnea surgery. And I suspect that the benefit is really due to the genioglossal advancement maneuver that is often part of the hyoid suspension. These are all things that are deserving of further study and clarification. This is a disease that’s only going to increase, since in America there’s an epidemic of obesity and we’re all aware of how difficult it is for most individuals to lose weight . I think we will see more of this in the future.
SLEEP DISORDERS FOR THE OTOLARYNGOLOGIST
Introduction
Although it was almost two centuries ago that Charles Dickens described a hypersomnolent, overweight child in the Posthumous Papers of the Pickwick Club, leading Sir William Osler to use the term “pickwickian”, the study of sleep and its disorders is still in its infancy. Although each human spends somewhere between a third and a half of his or her life asleep, little is known about why we need sleep.
In the last thirty years there has been a blossoming body of knowledge regarding the diagnosis and treatment of sleep disorders. The otolaryngologist is frequently involved in the management of these patients. A thorough understanding of sleep disorders and their treatment is key for the practicing otolaryngologist. One must realize, however, that since the field is relatively new there is little that is absolute, and what comprises standard of care today may change with the rapid evolution of the field.
Sleep
Sleep is a state with which we are all familiar but is poorly understood. Beginning with the advent of the EEG, investigators began studying the electrical activity of the brain during sleep. Also, it was recognized early on that there are two distinct sleep states: a peaceful, physiologically restive state (non-REM or NREM sleep), and a different state characterized by muscle atonia and autonomic activity (increased heart rate and blood pressure) and rapid eye movements (REM sleep). Using the EEG investigators have divided NREM sleep into 4 stages. Stage I is the lightest and stage IV is the deepest. Through the night a person cycles up and down through these stages, with REM sleep occurring intermittently between stage II and I. As the night goes on more time is spent in REM sleep. This is important to realize, as the
obstructive sleep apnea syndrome is more likely to occur during REM sleep, and thus a partial night sleep study may miss clinically important sleep apnea if done in the first portion of the night.
Sleep Disorders
In 1990 authorities published the International Classification of Sleep Disorders. This identifies four categories of sleep disorders: dyssomnias, parasomnias, medical/psychiatric sleep disorders, and proposed sleep disorders.
Dyssomnias are disorders characterized by inability to fall asleep or excessive sleepiness. These are divided into intrinsic, extrinsic, and circadian rhythm disorders. Intrinsic disorders arise from within the body. Although extrinsic factors may contribute, they are alone insufficient for sleep disruption. This category includes obstructive sleep apnea syndrome, central sleep apnea syndrome, and periodic limb movement disorder, to name a few. Extrinsic dyssomnias arise from factors outside the body. This category would include sleep disorders related to stress or conflict, influence or drugs or alcohol, environmental influences such as noise, or poor sleep habits. Circadian rhythm disorders are rooted in an alteration in a person’s sleep pattern which is neither desired or normal. An example of this would be jet lag.
Parasomnias are characterized by inappropriate central nervous system activity during sleep. These are divided into arousal disorders, sleep/wake transition disorders, parasomnias associated with REM sleep, and other parasomnias. An example of an arousal disorder is sleepwalking, whereas sleep talking is considers a sleep/wake transition disorder. REM sleep parasomnias include nightmares, whereas other parasomnias include bruxism, enuresis, and primary snoring.
Medical /Psychiatric sleep disorders are divided into those associated with mental disorders, those with neurologic disorders, and those associated with other medical illnesses. This is a heterogeneous group of disorders that involve sleep disturbance.
Proposed sleep disorders are those disorders which have been suggested to be distinct clinical entities, but insufficient data exist currently to fully affirm or refute their existence. Presumably an individual disorder will be recategorized or removed as further research is performed. Sleep-related laryngospasm is one such disorder which may interest the otolaryngologist.
Sleep Disorders for the Otolaryngologist
The primary sleep disorders that are brought to the attention of the otolaryngologist are those referable to upper airway obstruction. In a child this most often is related to adenotonsillar hypertrophy. In adults patients may present for a variety of reasons, or they may be incidentally picked up while being seen for other reasons. Diagnosis and management will be discussed below.
The pathophysiology involves obstruction of the upper airway during sleep. This is often associated with snoring, although snoring may or may not be associated with airway obstruction. As the airway obstructs the patient becomes hypercapnic and hypoxemic. This leads to an sub-wakeful arousal which opens the airway. Thus, the obstruction is relieved but at the expense of sleep fragmentation. The patient therefore has non-restorative sleep.
Definitions
To understand the specific disorders one must understand some of the definitions. An apnea is defined as cessation of airflow for ten seconds which results in an arousal. If the chest wall continues to mechanically move during this time, then it is an obstructive apnea. If the chest wall does not attempt to ventilate, then it is presumably due to a neurologic etiology and is termed a central apnea. Sometimes there are characteristics of both an obstructive and a central apnea, and this is termed a mixed apnea. The number of apneas per hour is termed the apnea index.
A hypopnea is a less well-defined entity, but usually is considered a diminution in airflow which results in hypoxemia and results in an arousal. The number of hypopneas per hour is termed the hypopnea index.
Functionally, there is little difference between apneas and hypopneas, and the sum of these vents per hour is termed the apnea-hypopnea index (AHI). This is also referred to as the respiratory disturbance index (RDI). Occasionally a lab will also report the arousal index, which is the number of arousal per hour. This may be different than the RDI due to limb movement or other causes of arousal.
Generally the obstructive sleep apnea syndrome (OSAS) is considered to be an RDI > 5. Also described is the obstructive sleep hypopnea syndrome (OSHS) which is a hypopnea index of greater than 15, but as mentioned there is little clinical utility in differentiating this from OSAS. Severity of OSAS is also stratified by the RDI, with mild being considered 5-20, moderate 20-40, moderate-severe 40-60, and severe > 60.
As the field of sleep medicine progressed there arose an awareness of certain patients who report excessive daytime sleepiness but do not have OSAS (e.g. RDI < 5). By esophageal manometry some of these patients have been shown to have increased negative thoracic pressure during inspiration. Thus, their increased work of breathing is thought to be responsible for their symptoms. This syndrome has been termed the upper airway resistance syndrome (UARS). Together with OSAS these are jointly referred to as sleep-disordered breathing (SDB). Those patients who snore but have an RDI < 5 and who do not have increased intrathoracic pressure upon inspiration simply have primary snoring. One hypothesis is that these disorders represent a spectrum of disease with primary snoring being the mildest, followed by UARS, and finally OSAS as the full manifestation of the disease.
Pathophysiology
Although incompletely understood, the pathophysiology of OSAS relates to airway collapse. This may occur at various levels, including the palate, the base of tongue, and the hypopharynx. Nasal obstruction appears to facilitate or exacerbate the syndrome although it does not appear to be primarily responsible. Unfavorable anatomy appears to be the most important cause. This can be due to a narrow palate, an elongated uvula, redundant tissue at the base of tongue, micro/retrognathia, a retrodisplaced hyoid, and so on. Adenotonsillar hypertrophy may be a cause as well, particularly in the pediatric population. Experimental evidence shows that in the pharynx the collapse occurs predominantly from the lateral walls, not merely from anteroposterior collapse as might seem likely in patients with elongated palates. This also may explain the way in which obesity increases the prevalence of OSAS, as the lateral pharyngeal fat pads may narrow the airway in the lateral dimension.
Although unfavorable anatomy is important etiologically, there also appears to be a physiologic defect in the pharyngeal dilators. There is also experimental evidence that longitudinal tension appears to be inversely related to airway collapse. Additionally, extrinsic factors such as sedating medications may exacerbate the physiologic defects.
Why treat OSAS?
Untreated, OSAS has a rather impressive list of deleterious consequences. For example, the incidence of systemic hypertension and its attendant sequelae is much higher in these patients. It is thought that this is due to systemic catecholamine release. Better understood is the link to pulmonary hypertension and cor pulmonale. The lung shunts blood away from hypoxic areas, presumably as a evolutionary efficiency mechanism during times of regional hypoxia (i.e. mucus plugging). However, during chronic episodic total lung hypoxemia the entire pulmonary vasculature constricts, and ultimately right-heart failure ensues. Other abnormalities associated with OSAS include myocardial arrhythmias, coronary artery and cerbrovascular disease and polycythemia. Whether due to previously mentioned problems or due to an independent cause, patients with OSAS have been shown to have an increase in mortality. Lastly, the neurocognitive compromise associated with inadequate sleep has been linked to poor performance and increased rates of industrial and traffic accidents.
Evaluation
History
Frequently a patient’s bed partner may give more important information than the patient. Loud snoring and observed apneas are key historical points. Epidemiologically OSAS occurs more commonly in obese people, in men, and with increasing age. However, absence of these factors clearly does not exclude the possibility of sleep apnea. One should try to get a feel for the amount of fatigue a person experiences, as well as questions about sleep hygiene, such as amount of nighttime alcohol and caffeine. Persons with insomnia should be questioned about reading in bed or watching TV in bed, as these are thought to contribute to insomnia. Morning headaches and impotence may be a tip-off for OSAS. Lastly, the examiner should always be vigilant to the possibility of malignancy and investigate questions related to recent changes in symptoms, weight loss, and symptoms such as otalgia, dysphagia, dyspnea, and hoarseness.
A questionnaire is also frequently administered to the patient in order to ascertain the patient’s subjective level of fatigue. One such tool commonly used is the Epworth Sleepiness Scale. In this scale a patient rates the chance of dozing off in eight different settings and a numerical score is tabulated. This may help identify patients in whom the subjective level of fatigue is discordant with the RDI, such as those patients with UARS.
Physical
Frequently the OSAS patient is obese. Neck size over seventeen inches has been shown to be a risk factor for OSAS. In someone with a neck over seventeen inches who snores the incidence of OSAS has been quoted at 30%. As part of the vital signs it is a good idea to calculate the body-mass index (BMI), which is obtained by dividing the weight in kilograms by the square of the height in meters. Additionally, one should check the blood pressure, as the prevalence of hypertension is very high in this population.
The rest of the physical exam should focus on sites of potential obstruction in the head and neck. In particular, one should look at the tonsils, the length and width of the soft palate, size of the tongue, and position of the jaw and hyoid. Nasal obstruction should be looked for, as well as dynamic nasal collapse. As always, a complete head and neck exam should be performed and the possibility of tumor excluded.
M..ller’s maneuver is designed to look for thee site of collapse. With the flexible scope in position, the patient tries to inspire against a closed mouth and pinched nostrils. The inspiratory forces then serve to collapse the airway. By doing this one can get an idea of the relative collapsibility of the palate as compared to the base of tongue or the hypopharynx. Although not terribly sensitive or specific, it still can be a helpful diagnostic and decision-making aid and adds little effort to the physical exam.
Testing
The gold standard for assessing OSAS is the polysomnogram, or sleep study. Although not completely standardized, the typical polysomnogram (PSG) will have measurements including an electroencephalogram (EEG), electro-oculogram, submental and tibial electromyogram (EMG), nasal or oral airflow, respiratory movement or effort, oximetry, electrocardiogram (EKG), and sleeping position. Some may also include measurements of penile tumescence and multilevel esophageal manometry.
Some controversy exists about a split-night PSG. For economic and convenience reasons, frequently in the first half of the night a standard PSG is performed followed by PSG with CPAP titration during the second half (see Treatment: Non-surgical, below). Due to changes in the sleep architecture during the night it is possible that OSAS might not be picked up during the first half of the night. Thus, a negative first half of the study does not exclude OSAS and a complete study should be done without CPAP titration. However, a positive first half study has been shown to be reliable for diagnosis of OSAS, and in studies looking at CPAP titration, no advantage was found in using the whole night’s data over the first half of the night. Thus a positive study in the first half of the night may go on to CPAP titration in the second.
Although efforts are underway to investigate limited polysomnograms or versions that patients can perform at home, the gold standard at this time is still the full PSG at a sleep lab.
Cephalometric studies have been used to study potential OSAS patients. Using plain films of the head, measurements of various parameters are done and compared to normative data in an effort to predict the likelihood of OSAS in a particular patient. Although potentially useful as a screening tool, these studies are not routinely done at our institution.
The multiple sleep latency test (MSLT) is another tool to study sleep disorders. During a person’s normal waking hours he or she takes four or five monitored naps separated by a few hours. The time to fall asleep, or sleep latency, is measured (abnormal being too quickly). This may reveal dysfunction in patients with otherwise normal PSGs. For example, this may reveal narcolepsy. Also, patients with UARS who have been treated with CPAP have been shown to have an improvement in their MSLT testing.
Lastly, any patient who is being considered for PSG should undergo thyroid testing, as thyroid dysfunction may cause similar symptoms and should be corrected prior to PSG testing. Also, many of the patients with OSAS will have cardiopulmonary dysfunction, and appropriate preoperative testing should be performed prior to any surgical intervention.
Treatment
Non-surgical
In obese patients one should consider weight loss. It is felt that there is a threshold level of weight for most individuals above which they experience symptoms and below which they do not. Also investigational tools such as computed tomography have demonstrated the increase in the airway with weight loss. Additionally, obese patients have many other medical benefits to gain from weight loss.
Sleep hygiene should be reviewed. Alcohol and sedating medicines may exacerbate OSAS. Patients with insomnia should be counseled to avoid nighttime caffeine and avoid activities such as reading or watching television in bed. Lastly, since OSAS is usually worse when supine, some patients may benefits from relatively simple measures such as sewing a tennis ball into the back of a T-shirt to promote sleeping on the side.
Continuous positive airway pressure (CPAP) remains a mainstay of treatment. Via facemask or nasal mask, positive airway pressure is delivered. This prevents pharyngeal collapse and has been shown to alleviate PSG abnormalities and symptoms in many patients. If delivered at two different pressure levels (for inspiration and expiration) it is referred to as BiPAP. CPAP titration can be done, as mentioned previously, during the second half of a split-night sleep study. This is done to find the lowest level of pressure that alleviates the apneas. Despite its effectiveness, compliance is often an issue, as many cannot tolerate the mask or the high pressures necessary for certain individuals. Intolerance of CPAP is a common indication for surgery, and often the patient still requires CPAP after surgery but can tolerate it due to relief of nasal obstruction or the improved airway that requires lower pressures to achieve relief of OSAS.
Oral appliances are also effective in relieving OSAS. These work by mechanically moving the jaw or tongue forward and opening the airway. They have been shown to be effective, more so for mild or moderate OSAS. Although some find them difficult to use, in general compliance is superior to CPAP. No single appliance has been shown to be superior to another.
Surgical
When a patient opts for surgical therapy the otolaryngologist has a variety of considerations. Most would agree that prior to surgical intervention a PSG should be obtained. Some argue that medical therapy should be tried first with surgery reserved for medical failures. Many times, though, a combination of medical and surgical therapy is necessary for optimum results.
Anesthesia considerations are important in these patients. As many of them have cardiopulmonary issues, the appropriate pre-anesthesia testing is necessary. Also, many of these patients have the combination of a short, thick neck and some degree of retrognathia. This is a setup for an airway problem. Vigilant attention to intubation and extubation is necessary. Many anesthesiologists perform awake fiberoptic intubations and are very judicious in their use of medications which might compromise the airway. On the other end, it is recommended that extubation be done when relatively light. Of course, proper equipment for airway emergencies should be available. In this event, adjunctive measures such as the laryngeal mask anesthesia or trans-tracheal jet ventilation may buy the surgeon precious moments. As always, proper communication between the surgeon and anesthetist is important. Post-operative considerations include the possibility for post-obstructive pulmonary edema and the likelihood of post-operative hypertension. Depending on the individual patient, intensive care unit monitoring may be best for the first post-operative night.
If the obstruction appears to be at the palatal level, some palatal intervention is in order. The uvulopalatopharyngoplasty (UPPP) remains a mainstay of treatment. Described by Ikematsu in the 1950s for snoring, Fujita recognized in the 1980s that this was effective for the treatment of OSAS. In the procedure a portion of the soft palate, the uvula, and the tonsils are removed. The posterior pillars are sewn anterolaterally. Complications can include voice changes and oronasal regurgitation of food or liquids. Rarely, the devastating complication of nasopharyngeal stenosis can occur.
Also described for the palate is the laser-assisted uvulopalatoplasty (LAUP). While this can be done in the office, it often requires multiple sessions. It may also be more appropriate for primary snoring. A recent study, however, suggests that the short-term benefits reverse over time and, in fact, be detrimental.
For hypopharyngeal and base of tongue obstruction, first-line therapy at this institution is genioglossal advancement and hyoid myotomy and suspension (GAHM). In this procedure, a portion of the central mandible is advanced, and the inner table secured at the level of the outer table. This mechanically advances the tongue relative to the posterior pharynx. Also the neck is opened, and the hyoid bone is freed of its muscular attachments. It is then secured anteriorly by four sutures to the thyroid cartilage, moving the base of tongue forward. Also described for tongue base obstruction are procedures such as the lingual tonsillectomy, uvulopalatopharyngoglossoplasty, laser midline glossectomy, lingualplasty, and radiofrequency volumetric tissue reduction.
As mentioned before, although not specifically a direct cause of OSAS, nasal obstruction may be an aggravating factor in OSAS. Appropriate procedures such as septoplasty, turbinate reduction, and functional nasal reconstruction are appropriate adjunctive procedures. Additionally, nasal surgery may be necessary for some patients to tolerate their CPAP.
Worthy of mention is the Riley-Powell-Stanford surgical protocol. At their institution they have had success by performing UPPP and/or GAHM as first-line surgical therapy (phase I). This results in a 61% success rate. For patients with persistent disease, they then undergo maxillomandibular osteotomy and advancement (Phase II). This resulted in a 97% success rate in their patients who failed Phase I surgery. Success is defined as a 50% reduction in the RDI and an RDI of <20. Thus, maxillomandibular osteotomy and advancement may be a viable option for certain patients. At our institution, we involve the oral surgery service in the care of these patients.
Lastly, the definitive surgery for OSAS is tracheotomy, which results in upper airway bypass. This is usually reserved for morbidly obese patients. In these patients it is recommended by many to use the neck skin to line the tract, resulting in a more permanent tract. Tracheotomy in this patient population is technically difficult and is not free of complications.
Conclusion
Sleep medicine is an exciting, relatively new field that has emerged. The otolaryngologist has become a key figure in the diagnosis and management of sleep disorders due to his or her familiarity with the airway and the ability to intervene surgically. An understanding of the medical and surgical issues involved is necessary for the otolaryngologist to deal with this field which is rapidly evolving.
STRIDOR, ASPIRATION, AND COUGH IN CHILDREN
Stridor 
Introduction
Stridor is a harsh, high-pitched musical sound produced by turbulent airflow through the upper airways. The evaluation must be tailored to the clinical situation, which may range from mild illness to a severe and life-threatening situation.
The larynx has 4 basic functions: ventilation of the lungs, protection of the lungs during deglutition, clearance of secretions by vigorous coughing, and vocalization. An infant’s survival depends upon the neurologic and structural integrity of the larynx.
Poiseuille’s Law dictates that flow through a cylinder is proportional to the radius to the fourth power. In the healthy neonate, the length of the glottis is 7 mm with a width of 4 mm.
The subglottis ranges from 4.5-7 mm in diameter. A narrowing of the subglottis by 1 mm can increase airway resistance 16-fold while decreasing cross-sectional area by 75%.
Bernoulli’s Law dictates that, as velocity increases through a constant area, pressure on the lumen wall decreases, thus encouraging collapse of the airway.
There are three zones of the airway characterized by stridor during different phases of respiration. Supraglottic obstruction results in a high-pitched, inspiratory stridor. Obstruction of the extrathoracic trachea, including the glottis and subglottis, is characterized by biphasic stridor with an intermediate pitch. Obstruction of the intrathoracic trachea (including first and second order bronchi) results in expiratory stridor (wheezing). This last area of obstruction is associated with retraction of the sternum, costal cartilage, and suprasternal tissue.
In contrast to stridor, stertor is a low-pitched inspiratory sound produced by nasal or nasopharyngeal obstruction.
History and Physical Exam
Immediate assessment of the urgency of the situation is vital. As with wheezing, decreased intensity of stridor may indicate either resolution or exhaustion and impending respiratory collapse.
Croup is the most common cause of acute stridor. Laryngomalacia represents the most common cause of congenital chronic stridor.
Croup tends to cause edema most markedly in the subglottis because the respiratory mucosa is loosely attached there.
Birth injury or neurologic abnormalities may suggest TVC paralysis, while a history of intubation suggests subglottic stenosis.
On exam, the child should remain with the parent while the examiner determines the degree of distress. Flaring of the nasal alae and the use of accessory neck and chest muscles are clues to increased respiratory effort. Supraglottic obstruction with air hunger will often cause the patient to sit with the neck hyperextended to improve airflow.
Once one has established that the child is well oxygenated and stable, the exam can proceed. Auscultation over the nose, mouth, neck, and chest can localize the site of obstruction and correlate the stridor with the respiratory phase. Glottic and supraglottic obstruction prolongs inspiration, while bronchial obstruction prolongs expiration.
Positional stridor most frequently results from laryngomalacia, micrognathia, macroglossia, or vascular compression. In all of these cases, improved airflow occurs when the baby is prone with the neck extended.
A weak cry points to either a disorder of the TVC’s or poor pulmonary function. While voice changes suggest a laryngeal lesion, a normal voice does NOT rule out a laryngeal cause of stridor. For example, bilateral TVC paralysis is usually associated with a normal voice.
If the stridor is present at birth, one can open the neonate’s mouth and pull the mandible and tongue forward. If the stridor lessens, then the obstruction is at the level of the larynx or higher. Passage of a nasal catheter can determine the patency of the nasopharyngeal airway.
The placement of an oral airway will bypass the obstruction in choanal atresia. In Pierre-Robin sequence, the placement of a nasopharyngeal airway will maintain the airway until a long-term decision is made, e.g., tracheostomy, mandibular distraction, etc.
One must ALWAYS maintain a high index of suspicion for an aspirated foreign body. In addition, one must remember the possibility of an upper esophageal foreign body that can also result in airway obstruction.
Transnasal flexible endoscopy should be performed in all stable stridorous patients while the patient is awake. Of note, one can also evaluate swallowing this way. While passing a flexible scope it is possible to rule out pyriform aperture stenosis, choanal stenosis, and adenoid hypertrophy. It is also possible to assess hypopharyngeal tone and TVC mobility while looking for signs of laryngomalacia and/or reflux. Occasionally, one may catch a glimpse of the subglottis.
Imaging and Further Workup
Lateral and A/P neck films are usually the first step in imaging. Inspiration is important on the lateral view; it distends the hypopharynx with air and places the epiglottis in a vertical position while stretching the A-E folds diagonally. Barium swallow is useful to detect aspiration, posterior laryngeal cleft, TEF, or external compression of the airway due to vascular lesions; it can also pick up non-radioopaque esophageal foreign bodies. Of note, it is very difficult to distinguish between posterior laryngeal cleft and garden variety aspiration on barium swallow. The most common extrinsic compressive disorder resulting in stridor is a double aortic arch. This may also be detected on barium swallow as bilateral curvilinear indentations at approximately the level of T-4.
In contrast, a pulmonary artery sling (aberrant left pulmonary artery) results in compression of the right mainstem bronchus and lower trachea. By barium swallow, this will result in an anterior indentation of the upper thoracic esophagus on LATERAL projection. An aberrant subclavian artery, though far less common, can result in the same finding on barium swallow.
CT and MRI may also be useful in suspected cases of vascular compression. CT is very useful in confirming extrinsic compression of the airway by mass effect, particularly in the case of retropharyngeal soft tissue swelling. MRI is superior to angiography in the diagnosis of vascular rings because it images airways and vessels simultaneously. The downside of MRI is that it requires general anesthesia or prolonged sedation. Thus, MRI is best used as a second line when echocardiogram or plain films/barium swallow have not determined the cause of stridor but have suggested a vascular anomaly. The weighting of choice is T1 spin echo with cardiac gating. In the near future (according to the radiologists) ultrafast imaging techniques may minimize the need for sedation and make MRI a far more commonly used modality.
Interestingly, Pickhardt found that completely normal A/P and lateral views of the chest essentially rule out a vascular ring. Obviously, radiographs play no role in critical cases of acute stridor.
Of note, ultrasound is an easy and inexpensive way to follow subglottic masses such as a hemangioma.
Airway fluoroscopy is a relatively new modality that has the benefit of being a dynamic study capable of evaluating multiple sites of obstruction simultaneously. Fluoroscopy usually involves 1-2 minutes of radiation exposure at doses of 4-7 mR/min; the average total dose is 10 mR. A thorough fluoroscopic examination involves evaluation of movement of the hemidiaphragms, observation for focal air trapping, and imaging of the airway from the nasopharynx to the mainstem bronchi in the A/P, oblique, and lateral projections. The most common diagnoses established by airway fluoroscopy are subglottic stenosis, laryngomalacia, and airway foreign body. Rudman found that nasopharyngoscopy was far better at detecting supraglottic and glottic lesions, while fluoroscopy was far better at detecting subglottic, tracheal, and bronchial lesions. Plain films were far less sensitive than either nasopharyngoscopy or fluoroscopy regardless of the site of lesion. Furthermore, fluoroscopy is 90% sensitive for the detection of bronchial foreign bodies, but only 32% sensitive in the detection of tracheal foreign bodies. Interestingly, fluoroscopy was initially used in the diagnosis of obstructive sleep apnea; it is as good or better than nasopharyngoscopy at detecting dynamic oropharyngeal collapse. In addition, fluoroscopy is better than rigid laryngoscopy and bronchoscopy at detecting tracheomalacia.
Rudman concluded that the combination of nasopharyngoscopy and airway fluoroscopy without plain films is the most cost-effective way to evaluate stridor in children (prior to rigid endoscopy).
Usually the history and physical exam, flexible endoscopy, and imaging will result in the correct diagnosis. Diagnostic rigid endoscopy is the gold standard; it is needed when the diagnosis remains in question, the evaluation suggests subglottic stenosis, a second distal airway lesion is suspected, or there is suspicion of a foreign body. While rigid and flexible endoscopy are complementary, rigid endoscopy represents the only means of safely examining the airway distal to the larynx.
Endoscopy
Atropine is useful both to decrease salivary secretions and to minimize the risk of bradycardia. The appropriate dose of atropine is 0.02 mg/kg with a minimum dose of 0.1 mg and a maximum of 0.5 mg/kg. Lidocaine can be applied topically to the larynx to prevent laryngospasm.
The appropriate equipment must be readied before starting the case. One should have an age-appropriate endotracheal tube on hand (4+age/4) and a size smaller. It is important during the procedure to measure the size of the air passage (especially the subglottis) using either an endotracheal tube or a bronchoscope. Inspection of the anatomic and mucosal contour is also important. However, most importantly, one must maintain a channel to the lungs for ventilation.
The Myer-Cotton grading system uses endotracheal tubes to grade the severity of subglottic stenosis. One passes an endotracheal tube such that the second graduated mark is at the level of the true vocal cords. The tube should then be connected to the ventilatory circuit and the pressure valve closed. The appearance of bubbles around the tube or an audible air leak is noted. The individual’s appropriate endotracheal tube size is defined as the largest endotracheal tube that permits a leak at less than 30 cm of water. This is then compared to the age-appropriate endotracheal tube size to assign the Myer-Cotton grade.
After examination of the airway and intubation, esophagoscopy or nasopharyngoscopy can be performed. In addition, the TVC’s may be observed during emergence from sedation.
Of note, a recent survey indicated that 72% of pediatric otolaryngologists have incorporated flexible bronchoscopy into their armamentarium. Obviously, a flexible bronchoscope does not allow for ventilation, but it has the advantages of a broad viewing field while allowing for inspection of the peripheral airways. It can also be manipulated through a tracheostomy tube or stoma. The most common indications for flexible bronchoscopy include the diagnosis of stridor in neonates, removal of secretions, inspection of the airway for trauma, and bronchoalveolar lavage. Pulmonologists have even been using the flexible scope alone for extraction of airway foreign bodies. In cases where the foreign body cannot be retrieved through a rigid bronchoscope due to distal migration, the flexible scope can be inserted through the rigid bronchoscope while maintaining ventilation (similarly, the flexible scope may be passed through an LMA or an endotracheal tube 4.5 or larger). For grasping, one can use ureteral stone forceps passed through the suction channel of a pediatric flexible scope or alongside a neonatal flexible bronchoscope. This requires an assistant to control the forceps.
The overall complication rate for rigid bronchoscopy is approximately 1% and 0.3% for flexible bronchoscopy. The most common complications in descending order are arrhythmia, laryngospasm, pneumothorax, and hemorrhage.
Post-extubation Stridor
Risk factors for post-extubation stridor include endotracheal tube size, the presence of a cuffed tube, the duration of mechanical ventilation, and the presence of underlying airway disease.
The air leak test has been widely used to predict when it is safe to extubate a patient. The test involves using a manometer to measure the minimum amount of air pressure required to produce an audible rush of air around the endotracheal tube as auscultated through a stethoscope placed over the larynx. If the leak only occurs with pressure greater than 20 mm Hg, then the patient has failed the test.
In children with laryngeal edema (e.g., croup) or recent tracheal surgery, the air leak test has been universally successful in predicting a good extubation outcome. In addition, the use of decadron has been effective in facilitating extubation in croup patients. However, there is no clear benefit to steroids in preventing post-extubation complications in normal airways. To date, all prospective studies of steroids in children in this setting have involved decadron; no other steroids have been studied.
Mhanna found that, while the air leak test works for children older than 7 years, it does not work for children with normal airways younger than 7. In general, children younger than are far more likely to fail extubation than older patients. Furthermore, Mhanna found that cuffed vs. uncuffed tubes made no difference as far as the incidence of post-extubation stridor in children less than 7, which confirms findings from previous studies. Current guidelines recommend the use of uncuffed tubes in children less than.
Epiglottitis
In children, the peak incidence of epiglottitis occurs in the 1-3 year old age group. A review of 407 cases of epiglottitis over 18 years in Rhode Island indicated a dramatic decline in the incidence of epiglottitis among children during the 1980’s, with 2/3 of cases occurring in adults. In children, the incidence went from 6/100,000/year to 0.3/100,000/year by 1992, while adults went from 0.78/100,000/year to 3/100,000/year over the same time period. There was no seasonal variation. Interestingly, no association was found with immunocompromise, though the relative risk of smoking was 2.3.
In children, the most common symptoms were difficulty breathing (80%), a history of fever (57%), and sore throat (50%). In adults, the most common symptoms were sore throat (91%), difficulty swallowing (82%), and difficulty breathing (37%). Stridor occurred in 80% of children, but only 27% of adults. Overall, 86% of lateral neck films were diagnostic. 68% of children versus only 21% of adults received an artificial airway. Risk factors for airway compromise included respiratory difficulty, stridor, drooling, and H. influenzae bacteremia. Complaints of sore throat and difficulty swallowing were associated with a milder disease course. 12 deaths occurred as a result of epiglottitis during the study period – 9 were adults.
The important point is that epiglottitis is now rarely seen among children because of the widespread use of the Hib vaccine; it has become essentially an adult disease. H. influenzae is no longer the most common cause, and the disease process tends to be less localized to the epiglottis with less risk of airway obstruction. Interestingly, epiglottitis due to thermal injury associated with illicit drug use occurred in 4 patients. This is an important consideration, particularly in adolescent patients.
Nonetheless, management guidelines for epiglottitis have not changed. Severe symptoms mandate prompt rigid endoscopy. In the case of mild to moderate symptoms, the immediate introduction of an artificial airway in ALL children has significantly decreased the number of deaths associated with epiglottitis, but the same is NOT true for adults with mild to moderate symptoms. Unless severe signs or symptoms are noted, adults can be observed in the ICU without an artificial airway. However, deaths occurred during observation in the ER, on the floor, and even in the ICU, so a low threshold for airway placement must be maintained.
Regardless, all patients should be treated with a beta-lactamase resistant antibiotic. The use of corticosteroids remains controversial.
Aspiration
Introduction
Aspiration involves penetration of secretions or other material below the level of the true vocal cords. All normal, healthy individuals experience some clinically insignificant aspiration, especially during sleep. Aspiration becomes problematic when the aspirate cannot be cleared or when the aspirate is significantly toxic or voluminous. In children, any significant dysfunction of swallow will also impair the function of the respiratory tract. Possible complications of chronic aspiration include tracheitis, bronchitis, bronchospasm, pneumonia, and pulmonary abscess.
A swallow consists of 4 phases: the oral preparatory and the oral transit phases, both of which are voluntary; and the pharyngeal and esophageal phases, both of which are involuntary.
In infants, squeezing liquid from the nipple (suckling) is part of the oral transit phase.The swallow reflex begins at 16 weeks gestation and is important in maintaining amniotic fluid balance. Suckle begins in the second and third trimester, thus, suckling is generally poorly coordinated and ineffective in premature infants while the pharyngeal swallow is generally normal. By 34 weeks gestational age, the premie should be able to suckle feed, though the baby may still have trouble coordinating swallowing and breathing. Suckling is regulated in the medulla and pons. During suckling, peristaltic anterior to posterior tongue motion with compression of the tongue against the soft palate occurs, creating repetitive negative intraoral pressure alternating with nipple compression.
More voluntary control of swallowing becomes possible with maturation of the higher cortical feeding centers. Thus, the oral phase undergoes the greatest change with age, while the pharyngeal and esophageal phases change very little. Chewing normally begins at 6 months of age, achieving 40% of adult efficiency by 6 years.
The afferent limb of the swallow reflex includes the glossopharyngeal, trigeminal, and superior laryngeal nerves. The impulses travel to the swallowing center in the floor of the fourth ventricle. The efferent limb begins in the nucleus ambiguus of the medulla and descends via CN X.
There are 3 mechanisms of airway protection in a normal individual: the laryngeal sphincter, cough, and swallow. There are 3 tiers of the laryngeal sphincter: the epiglottis and AE folds, the false vocal folds, and the true vocal folds. The swallow helps by clearing material from the larynx and hypopharynx.
Of note, the swallow frequency of an adult during sleep is 1/60th that of a sleeping preterm infant, who swallows at a rate of 6 times per minute. Infants can further increase this swallowing 8-fold during apneic spells, which serves as a protective mechanism. Pathologic pulmonary aspiration is secondary either to an abnormality in swallowing or an abnormality in protection of the airway. The causes of aspiration may be either mechanical (i.e., an anatomic change in the upper aerodigestive tract) or functional (e.g., a neuromuscular disorder).
There are 3 different categories of aspirated material: orally ingested, oral and upper airway secretions, or regurgitated gastric contents. The aspiration of oral secretions may be referred to as primary or direct aspiration. The aspiration of gastric refluxate may be referred to as secondary or indirect aspiration. Premature spill from the oral cavity to the oropharynx, incoordination between oropharyngeal motility and glottic closure, ineffective glottic closure, and incomplete bolus transport may all lead to direct aspiration.
History/Risk Factors
Gastroesophageal reflux (GER) is the abnormality most commonly associated with chronic pulmonary aspiration. Pulmonary symptoms can result either from direct injury secondary to aspiration or from reflex mechanisms designed to protect the airway. Signs and symptoms of aspiration due to GER can be obvious or subtle including postprandial cough, regurgitation, emesis, bronchospasm, laryngospasm, central apnea, and bradycardia. In fact, the incidence of significant GER in patients with Acute Life-Threatening Events (ALTE’s) is much higher than in normal infants. Near-miss SIDS may represent GER with aspiration during sleep.
The classic triad of pathologic GER includes failure to thrive, vomiting, and recurrent aspiration pneumonia.
Other risk factors that predispose an infant to aspiration include a depressed level of consciousness, deficient swallow mechanisms, general sickness or debilitation, preterm birth, and scoliosis (predisposes child to GER). CNS and neuromuscular diseases frequently affecting swallowing and increasing aspiration risk include cerebral palsy, epilepsy, muscular dystrophy, and intestinal motility disorders.
The feeding history is often key in the evaluation of aspiration. Poor sucking habits or nasopharyngeal reflux during feeds suggest oropharyngeal dyscoordination and may indicate poor swallow coordination. Signs of poor sucking habits include the need for an enlarged nipple hole, frequent choking or gagging with feeds, and failure to thrive.
Respiratory symptoms during or shortly after feeding are highly suggestive of aspiration. Swallowing difficulties frequently arise at approximately 4 months of age as the swallow apparatus begins to elongate, resulting in less protection from aspiration. The soft palate touches the epiglottis until 3-4 months of age. While the gag reflex is strong at birth, it usually weakens by 6-7 months of age.
Prenatal and birth history may also suggest risk factors. For example, polyhydramnios can be associated with defects of the esophagus (e.g., esophageal atresia, ectatic esophagus, or tracheoesophageal fistula). A traumatic delivery may have resulted in recurrent laryngeal nerve injury. A history of upper airway instrumentation can result in pseudodiverticulum or pneumothorax.
One should find answers to the following questions during the history. Is there cough or choking during feeds? Does vomiting occur with the choking? Is there a nocturnal cough? Stridor? Has the child experienced apneas? Is there a hoarse cry?
In addition, existing conditions that may be exacerbated by chronic aspiration include recurrent pneumonia, bronchitis, CF, asthma, failure to thrive, bronchopulmonary dysplasia, ALTE’s, and CHF.
Physical Exam
One should inspect (and palpate when possible) all mucous membranes of the nose, oral cavity, and oropharynx. It is also helpful to observe the child during feeding, and one can auscultate the lungs before and after a feed. The observation of nasopharyngeal reflux during feeding indicates swallowing dysfunction and is often associated with aspiration. One should place a finger in the mouth to evaluate the strength and coordination of the suck as well as resistance to deep finger insertion. Neurologic signs and symptoms, such as drooling or excess secretions in the mouth, may also become apparent. In addition, gaseous abdominal distention may be a sign of a tracheosophageal fistula (TEF).
The examination should include laryngoscopy and evaluation of the cranial nerves.
Workup
Lateral neck and plain chest films are typically the first step. A review of chest x-rays taken of children with chronic aspiration revealed 41% with localized infiltrates, 27% with diffuse infiltrates, 18% with bronchial wall thickening, and 14% with normal films.
A modified barium swallow (MBS) is valuable and is capable of distinguishing between aspiration that occurs directly at the time of the swallow and delayed aspiration occurring during the respiratory cycle but after the swallow. The MBS demonstrates oral, pharyngeal, laryngeal, and esophageal anatomy and can show oral motor dysfunction, pharyngeal incoordination, nasopharyngeal reflux, laryngeal penetration/aspiration, GER, and hiatal hernia. A barium swallow may also detect a laryngotracheal cleft, TEF, vascular ring, esophageal atresia, esophageal stricture, pyloric stenosis, or malrotation, and it may also pick up GER or a hiatal hernia. However, a barium swallow is only 50-85% sensitive and 70-75% specific for reflux.
Other studies which may be helpful include a 24-hour pH probe, gastric scintiscan, and esophagoscopy with or without biopsy. The scintiscan is the study of choice for determining the rate of gastric emptying. Esophageal manometry is difficult to perform in a nonsedated child and is not often used.
The pH probe is the gold standard for the diagnosis of GER. A reflux episode is defined as a drop in the esophageal pH to 4 or less. The number of episodes, as well as the number of episodes lasting more than 5 minutes, are factored in to determine if the reflux is clinically significant (a Euler-Byrne score >50). However, establishing GER does NOT establish aspiration.
A CT or MRI of the brain is useful if CNS problems are suspected. Direct laryngoscopy, esophagoscopy, and bronchoscopy are necessary if the history, physical exam, and above tests do not reveal a cause of chronic aspiration. Endoscopy can assist in the diagnosis of a laryngotracheal cleft, reflux esophagitis, and vascular rings.
Treatment
If anatomic abnormalities such as a cleft or TEF exist, then they must be corrected surgically. If an endotracheal or tracheostomy tube is present, then it should be removed as soon as it is safe to do so.
The natural history of GER in infants is spontaneous resolution by 18 months to 2 years of age, so the treatment of GER begins with positioning, thickening of feeds, the use of small and frequent feeds, and fasting before bedtime when appropriate. The optimal position for feeding in infants is prone and flat with the entire body tilted 30 degrees postprandially. This position decreases GER, increases gastric emptying, decreases aspiration, and decreases energy expenditure. Sitting may actually exacerbate reflux. Though generally recommended, there is little clinical evidence to support thickening of feeds or smaller, more frequent feeds. If more conservative measures fail, then medication should be tried. Metoclopramide increases LES tone and gastric emptying. H-2 blockers and PPI’s decrease the acidity of the refluxate. Sucralfate can be used in GER related to delayed gastric emptying from ulcers in or near the pylorus. Failure of 6 weeks of antireflux medications is an indication for antireflux surgery.
Of note, the pH of gastric secretions varies among infants. 1/3 of premies produce little or no gastric acid at birth. Chemical pneumonitis occurs at a pH less than 2.4, while the pH of most formulas is approximately 5. This emphasizes the importance of promotility agents, rather than acid suppression, in the treatment of GER in young children.
In the setting of neurologic problems, one must minimize the complications associated with aspiration. Broad antibiotic coverage against anaerobes should be used in cases of aspiration pneumonia. Good oral hygiene and swallowing therapy are also helpful. If the aspiration is self-limited, one can try an NG tube, a G tube, parenteral feeding, or a tracheostomy until the patient is better.
As far as surgery, a feeding gastrostomy or jejunostomy tube is the most common procedure for severe or irreversible swallowing dysfunction. Gastric fundoplication for severe GER unresponsive to medications will help, but it won’t prevent the aspiration of oral secretions. Cricopharyngeal myotomy is appropriate for patients with cricopharyngeal achalasia. A tracheostomy represents a temporary means of increasing pulmonary toilet, but alone it is not adequate in the setting of chronic aspiration. Vocal fold medialization will help in the presence of a paralyzed TVC. However, in the case of congenital recurrent laryngeal nerve paralysis, surgical procedures on the larynx should be postponed since the nerve function will often recover.
The goal of treatment in chronic aspiration is to decrease the number of episodes of aspiration pneumonia, prevent acute and chronic bronchopulmonary complications, decrease hospitalization and nursing care requirements, and improve the quality of life for both the patient and the family. Laryngeal diversion and separation represent the most definitive procedures for chronic aspiration. These procedures are indicated for severe aspiration due to laryngeal incompetence that is NOT expected to recover (e.g., central neurologic deficits). Though it is theoretically possible for these patients to learn esophageal speech, phonation generally does not occur.
Examples of these procedures are the Lindeman and modified Lindeman procedures.
At the Children’s Hospital Medical Center in Cincinnati, they rarely perform laryngeal diversion or separation. In cases of chronic aspiration, the children are fed strictly through a gastrostomy or jejunostomy tube. If reflux is present, they also obtain antireflux surgery. That then leaves the possibility of aspiration of oral and oropharyngeal secretions. Thus, they perform bilateral submandibular gland excision with bilateral parotid duct ligation, which decreases saliva production and aspiration while preserving voice. This also has the advantage of decreasing/eliminating drooling in neurologically impaired children. This almost always obviates the need for a tracheostomy, but a trach can still be performed in those patients who continue to aspirate (rare). In their series of 16 patients, complications were uncommon and included acute parotitis, sialocele requiring intraoral excision, and one case of chronic parotitis requiring superficial parotidectomy. No patients had problems with increased dental caries or needed to change their dental care. Their workup of children with chronic aspiration typically consists of a MBS, FEEST, and 24-hour esophageal pH probe.
Airway foreign body
Children less than 3 years old have a tendency to put things in their mouths. TheNational Safety Council (1984) determined that foreign body aspiration was the fourth leading cause of accidental death in children age 1-3 years, and the third leading cause of accidental death in infants less than 1 year. Foreign body aspiration is twice as common in boys. It is important to acknowledge that esophageal foreign bodies can also cause symptoms of respiratory compromise via compression of the trachea. This occurs in up to 10% of cases of esophageal foreign body. In the case of caustic ingestion, the presence of respiratory distress is highly predictive of severe digestive lesions.
Vegetable matter is the foreign material most commonly found in the pediatric airway (70-80%). Nuts are the most common, followed by carrot pieces, beans, sunflower seeds, and watermelon seeds. The second most common class of aspirated foreign material is plastic (5- 15%).
As far as airway foreign bodies resulting in death, 2/3 involve toys intended for children’s use. The most common object causing death is a balloon (29%), followed by balls (13%) and marbles (6%). At least two deaths have been reported due to choking on examination gloves given to children in clinician’s offices. Aside from conforming objects, spherical objects are the most likely shape to cause choking when aspirated.
The most common site to encounter an airway foreign body is the right mainstem bronchus. Foreign bodies will be found lodged in the bronchi 80-90% of the time.
The classic story involves a brief period of choking, gagging, or wheezing that may be associated with hoarseness or aphonia. Coughing and/or choking is highly suggestive of a foreign body, while respiratory distress is rare. One must always remember the axiom that, “A positive history must never be ignored, while a negative history may be misleading.” Signs and symptoms of foreign body aspiration may mimic asthma, croup, and pneumonia. New onset of wheeze in a previously healthy child should heighten suspicion.
The natural history of an airway foreign body involves 3 stages. First, there is a choking episode followed by coughing, gagging, and occasionally complete airway obstruction. This is followed by an asymptomatic interval as the protective reflexes fatigue and irritation subsides, which contributes to a delay in diagnosis (20-50% of cases aren’t diagnosed until more than week after the initial aspiration). Finally, signs and symptoms of complications manifest with cough, hemoptysis, pneumoia, lung abscess, fever, or malaise. Foreign bodies in the larynx or cervical trachea tend to cause inspiratory or biphasic stridor. A prolonged wheeze on expiration suggests involvement of the intrathoracic trachea. A discrepancy between the two sides of the chest on auscultation suggests involvement of a mainstem bronchus. The classic triad of unilateral wheeze, cough, and ipsilateral decreased breath sounds occurs in less than ½ of cases.
Of note, disc batteries contain a high concentration of KOH and NaOH (base, NOT acid), and therefore cause liquefactive necrosis. They begin to cause mucosal damage as early as one hour post-ingestion.
In the setting of a suspected foreign body, inspiratory/expiratory chest films or lateral decubitus films are important. Lateral decubitus films are more useful in younger children who cannot cooperate with the timing of the respiratory cycle. Suggestive findings include air trapping, infection, atelectasis, and failure of the mediastinum to shift when the involved lung is dependent. Inspiratory hypoinflation and expiratory hyperinflation are the hallmarks of a bronchial foreign body. It is important to remember that most airway foreign bodies are radiolucent. However, chest x-ray and exam are commonly normal in the first 24 hours following foreign body aspiration. Thus, history is the most important aspect of the diagnosis, and endoscopy is frequently performed on the basis of a suggestive history, alone. The key to treatment is recognition of a person in acute airway distress. Complete airway obstruction – indicated by an inability to speak or cough — is an absolute emergency. Blind finger sweeps are contraindicated because they may further impact the foreign body. Back blows may also impact a foreign body resting below the TVC’s in the larynx. Nonetheless, back blows are the appropriate first step in children less than 1 year. In children older than 1 year, gentle abdominal thrusts in the supine position are appropriate. In older children and adults, the Heimlich maneuver is appropriate.
Most of the time, an airway foreign body is not a dire emergency, and endoscopic removal can be scheduled when trained personnel are available, the instruments have been checked, and the techniques have been tested. In addition, one must remember that multiple foreign bodies occur in 5-19% of cases.
Cough
Introduction
Cough represents the most common symptom of respiratory tract disease. The majority of diseases in the first decade of life are respiratory in origin. Respiratory illness accounts for 2/3 of all infections in the first 5 years of life. Cough, in general, is less vigorous in newborns and premature infants.
The respiratory system basically has 4 protective mechanisms: cough, the gag reflex, the mucociliary escalator (which represents the normal clearance mechanism in healthy individuals), and the phagocytic/lymphatic systems. The two functions of cough are to expel foreign material from the airway and to remove excessive secretions from the airway. Cough becomes a factor only when there is an abnormal type or quantity of material to be removed from the airway, or when the mucociliary escalator is ineffective.The stimuli of cough may be categorized into 4 groups: chemical (e.g., cigarette smoke), mechanical (e.g., vascular rings), thermal (e.g., cold dry air), and inflammatory (e.g., increased mucus).
The afferent pathway of the cough reflex begins with cough receptors, of which there are 4 classes. The slowly and rapidly adapting receptors respond to tactile stimulation and are most numerous at the level of the carina and large bronchi. The C-fiber receptors respond to chemical and mechanical stimulation and are present within the mucosa from the larynx to the alveoli.
The pulmonary stretch receptors respond to mechanical forces and are present within the smooth muscle of the airway. The highest cough receptor concentration occurs at the larynx, the lower half of the trachea, the carina, and the mid-sized bronchi. The carina is the single most sensitive site.
Upon stimulation of the cough receptors, the signal passes via cranial nerves IX and X to the upper brainstem/pons. Of note, there is also cortical input such that cough can be initiated or suppressed in the awake patient.
The efferent pathway consists of cranial nerve X and spinal nerves C2-S2, which result in stimulation of the muscles of the diaphragm, pharynx, larynx, intercostals, abdominal wall, and pelvis.
A cough occurs in 4 phases. The inspiratory phase begins with gasping inspiration and ends with glottic closure. The contractive phase occurs with stimulation of the appropriate muscles resulting in contraction against a closed glottic/supraglottic sphincter. During the compressive phase, there is a marked increase in alveolar, pleural, and subglottic pressure. In the expulsive phase, rapid opening of the glottis results in the release of trapped air at flow rates that can reach as high as Mach 0.75 in the adult.
The laryngeal closure reflex is triggered by sensory input from the superior laryngeal nerve. The true vocal cords close first, followed by closure and down-turning of the false vocal cords, followed by closure of the aryepiglottic folds. The closure of the false vocal cords is actually the most important step in preventing premature air escape during cough.
Laryngospasm represents a maladaptive glottic closure reflex mediated solely by the superior laryngeal nerve. It can be triggered by tactile stimulation of the endolarynx or mechanical or chemical stimulation of the esophagus.
Increased airway secretions are necessary for an effective cough. However, glottic closure is not essential for cough. Intubated patients can cough, albeit with earlier and lower peak flow rates.
History
Age of the child is an important factor. Cough is unusual in neonates and suggests a congenital anomaly, gastroesophageal reflux (GER), cystic fibrosis (CF), or chlamydia pneumonia.
A chronic cough is variably defined as a daily cough greater than 2-3 weeks in duration.
Chronic cough affects from 7-10% of children, and its diagnosis rarely requires diagnostic procedures beyond the scope of the primary care physician except in cases of very young children. In the majority of cases, the diagnosis is based on a positive response to treatment.
Holinger found that in children 18 months old or younger, the three most common causes of chronic cough were aberrant innominate artery, cough variant asthma, and GER. In children age 18 months to 6 years, sinusitis (50%) and cough variant asthma were the most common causes. In children aged 6-16 years, cough variant asthma (45%), psychogenic cough (32%), and sinusitis were the most common causes. A similar study by a pulmonologist that did not break findings down by age found the most common cause of chronic cough in children to be mild to moderately severe reactive airway disease (59%), chronic sinusitis (10%), and GERD (10%). Involvement in daycare results in increased exposure to upper respiratory pathogens. In fact, recurrent viral upper respiratory infections (URI) represent the number one cause of both acute and chronic cough in children. Preschool age children average 8 or more episodes of URI in one year.
Seasonal variation suggests an allergic etiology. This diagnosis may be confirmed with a response to empiric therapy with an antihistamine. Malabsorption (i.e., poor growth despite a good appetite), rectal prolapse, and nasal polyps are all signs that point toward CF. In fact, the presence of nasal polyps in a child mandates a sweat chloride test. Most importantly, CF should be considered in any child with chronic cough.
Environmental factors may also play an important role. Cough not associated with a URI is more common in high pollution (e.g., urban) areas. In a large cohort study in Tucson, they found that prenatal smoking doubled the risk of wheezing (and, to a lesser extent, coughing) during the first 3 years of life. However, postnatal parental smoking is NOT associated with wheezing, coughing, or other respiratory symptoms during the first decade of life. Adolescents with cough must be suspected of smoking, themselves.
When taking a history, the specific features of a cough should be determined (quality, timing, duration, and productivity). In addition, establishing the immunization status is important. It is important to distinguish between a persistent cough, which is most typically caused by reactive airway disease or bronchitis, and a recurrent episodic cough, which is most commonly due to recurrent URI.
A barking cough suggests croup.
A frequent, repetitive, honking cough suggests a psychogenic cough. This is most common in adolescents and typically occurs throughout the office visit and has a disruptive effect. Importantly, this is the only type of chronic cough that is completely absent during sleep.
A paroxysmal cough with repeated coughs in quick succession followed by rapid inspiration (“whoop”) suggests pertussis, but an infant with pertussis may demonstrate no cough or a cough resulting in facial plethora or cyanosis; the paroxysm in an infant may terminate with vomiting or apnea. In fact, the whoop is uncommon in both infants and children older than 5 years. The paroxysmal stage usually lasts 2-4 weeks, and anywhere from 5-20 coughs may occur with a single expiration. Pertussis typically occurs in epidemic cycles every 2-4 years. 60% of cases occur in children less than 5 years old, and it represents the most frequently reported vaccine preventable disease among children in this age group. The whole cell vaccine was associated with adverse events, which decreased significantly with wide-spread use of the acellular pertussis vaccine in the 1980’s. Complications such as pneumonia or severe neurologic sequelae occur in 4-15% of patients with pertussis.
A staccato cough suggests chlamydia pneumonia. This typically occurs in the first 6 months of life, and is usually associated with a prolonged afebrile illness that involves congestion, cough, tachypnea, rales, hyperinflated lungs with diffuse interstitial infiltrates, peripheral eosinophilia, and increased serum immunoglobulins. Conjunctivitis may precede the cough. As in pertussis, the coughing spells may end with cyanosis and/or emesis.
A postprandial and bedtime cough suggests GER. If the cough is associated directly with feeding, this suggests an aortic arch anomaly, laryngotracheal cleft, or tracheoesophageal fistula (TEF). Nocturnal cough, alone, is consistent with GER, sinusitis, allergic rhinitis, or cough variant asthma.
It is unusual for a young child to expectorate sputum, even in the setting of a bacterial pneumonia, since the sputum is usually swallowed. In this case, a “productive” cough may present with vomiting, instead.
Hemoptysis is unusual in children. One should consider bronchiectasis, CF, airway foreign body, pulmonary hemosiderosis, and tuberculosis.
Physical Exam
Young children with pulmonary disease increase their tidal volumes by increasing their respiratory rate, thus, the rate is important to note. In addition, a decreased I:E ratio is strongly suggestive of asthma (or a lower airway process). A normal I:E ratio ranges from 1:2.5 to 1:3.
Listen with and without a stethoscope. A wheeze on forced expiration suggests asthma.
Stridor with cough suggests a partial upper airway obstruction.
Sniffing, throat clearing, and hyponasal speech suggest chronic nasal, sinonasal, or adenoid disease. ‘Nine’ is one of the few words in English with an obligate nasal sound. Thus, nasal airway obstruction can be tested for by having the patient repeat “ninety-nine” with the nose occluded and unoccluded to assess for hyponasality. In addition, the “nasal salute” suggests chronic nasal disease.
In addition, one should observe for rashes, allergic shiners, adenoid facies, and asymmetry of chest motion. On examination of the ears, a hair or cerumen touching the TM can cause cough. Posterior pharyngeal cobblestoning suggests nasal/nasopharyngeal disease.
Digital clubbing is typically seen in chronic suppurative lung disease (e.g., CF, bronchiectasis, and hypersensitivity pneumonia) but is rare in RAD/asthma.
Workup
All children with chronic cough should obtain a P/A and lateral chest x-ray (hopefully, this will have been done before the child reaches you). Other studies that may be obtained as directed by one’s history and physical include a sinus CT, a lateral neck film, a barium swallow, inspiratory/expiratory chest films (or lateral decubitus films in younger, less cooperative children), a sweat chloride test, a CBC with differential, eosinophil count, pulse oximeter, ABG, ESR, PPD, PFT’s with methacholine challenge, and a sputum sample. In CF, sputum is frequently purulent but rarely foul smelling. A Hansel stain represents a quick and easy way to identify allergy (>5% eosinophils).
According to Holinger, endoscopy was the most useful diagnostic tool in children younger than 18 months. Barium swallow and empiric treatment with bronchodilators were also useful in this age group. In the 18 month-6 year age group, sinus films were the most helpful followed by endoscopy and a trial of bronchodilators. In the 6-16 year old age group, PFT’s with a methacholine challenge were the most useful diagnostic tool, followed by sinus films.
To diagnose pertussis, a sample must be taken from the nasopharynx then grown on Regan-Lowe or Bordet-Gengou agar. The culture is approximately 80% sensitive – less if the patient is on antibiotics. A positive result must be reported to the health department. Both the patient and household contacts should be treated with 14 days of erythromycin or trimethoprim/sulfamethoxazole.
More Differential Diagnosis
One must always maintain a high level of suspicion for an airway foreign body. Cough represents the most common symptom of a bronchial foreign body, occurring in up to 94% of cases. Furthermore, esophageal foreign bodies may also produce airway symptoms, including cough, secondary to tracheal compression.
GER is a frequent cause of cough in the neonate/infant. GER is responsible for chronic cough in 10% of cases of children with normal chest x-rays.
Laryngomalacia typically presents with stridor, but 10% will present with cough.Usually there is a positional history (e.g., the cough occurs when the child is supine and with increased activity).
Postnasal drip as a cause of cough is controversial. Dye studies in the 1930’s demonstrated that secretions in the nasopharynx enter the esophagus and not the larynx.
Asthma classically presents with a wheeze, though it may present as cough variant asthma. In almost all children with asthma/RAD, the cough is exacerbated by exercise or exertion (e.g., running or laughing). The cough typically occurs with exertion and during sleep.
Most asthmatics develop asthma within the first five years of life. Furthermore, though asthma may present with cough alone, approximately 2/3 of children without a wheeze initially go on to develop classic asthma. The diagnosis of cough variant asthma may be diagnosed either via a trial of bronchodilator therapy – in which case respiratory symptoms should resolve – or via PFT’s with a methacholine challenge (the gold standard). Because the onset of asthma generally occurs before the age of 6, Callahan (Pulmonology) recommends that children older than 6 with a suspicion of asthma/RAD should undergo PFT’s. In contrast, children less than 6 with a suggestive history may be given a trial of bronchodilator therapy.
Chang is concerned about the trend towards equating chronic cough with asthma. The use of cough, alone, in the diagnosis of “cough variant asthma” has at least partly contributed to the marked increase in prevalence of asthma over recent years and may be resulting in a lot of overtreatment. She points out that cough in children, more often than not, will resolve spontaneously. Thus, it is difficult to determine the true benefit from a therapeutic trial, and this has been borne out in prospective studies. The treatment of children with cough alone using an inhaled steroid and a bronchodilator showed no benefit over placebo in decreasing cough frequency. These children may have increased cough receptor sensitivity, but they do not have asthma. In the lab, it has been established that the triggers for cough and wheeze are similar, but the physiologic pathways are distinctly different. Cough frequency does not correlate with airway caliber or FEV1. In the absence of wheezing or shortness of breath, persistent nocturnal cough has NOT been correlated with asthma. Chang concludes that a trial of asthma medications may be attempted, but the cough should respond within one week. If there is no response within a couple of weeks, the medication should be abandoned; it is of no use to try increasing the dose.
Bronchitis usually exists in association with other respiratory diseases. Frequently, the trachea is involved concurrently. The pathogen is usually viral. Common causes include influenza, measles, typhoid, pertussis, diphtheria, and scarlet fever. Bacterial infection, if present, usually occurs secondary to the viral infection; S. pneumoniae and H. Influenza are the most common culprits. Chronic bronchitis in children is unusual in the absence of an underlying pulmonary or systemic disease (e.g., CF, immotile cilia syndrome, etc.). Chronic bronchitis typically presents as a chronic, nonproductive cough occurring after a respiratory infection. It may last for weeks, but usually resolves within 10 days. It is exacerbated by a dry environment, thus fall and winter witness the highest incidence. The usual age of affected children is 5-7 years old. If the bronchitis lasts for more than 2 weeks, one must consider a bacterial infection (particularly pertussis), atelectasis, asthma, CF, an immunodeficiency, or a foreign body.
Bronchiolitis commonly occurs in the lower respiratory tract of children 2 years old and younger. The cause is usually RSV. The characteristic cough is paroxysmal and wheezy, and is frequently associated with tachypnea.
Bronchiectasis refers to a dilation of the bronchi due to inflammatory changes with accumulation of secretions primarily affecting the bronchial wall. Rarely, bronchiectasis can be congenital, but it is usually secondary to chronic pulmonary infections (most commonly CF).
Bronchiectasis may also be seen in the setting of GER. Bronchiectasis is characterized by a chronic productive cough with repeat episodes of pneumonia involving the same lung segment (often the left lower lobe). Hemoptysis occurs in 50% of patients, and one may also see clubbing. Of note, bronchiectasis is one of the components of Kartagener Syndrome (also situs inversus, otitis, and chronic sinusitis).
Mycoplasma pneumonia typically presents in school-aged children with paroxysms of cough during sleep.
TREATMENT OF LARYNGOMALACIA
Stridor is a harsh, high-pitched musical sound that results from turbulent airflow through the upper airway. Evaluation of stridor is dependent upon the clinical situation, as the underlying etiology may range from mild illness to a severe and life-threatening situation. When considering a pediatric patient, it should be noted that eighty-five percent of children under the age of 2.5 years presenting with stridor have a congenital etiology. Congenital stridor often times is not present at birth, but will typically present before the age of four months. The remaining cases are accounted for by inflammation, trauma, or foreign bodies. Pediatric stridor has a variable age of onset. The typical patient usually presents with a sudden onset of symptoms. As a basic guideline, acquired stridor is more likely than congenital stridor to require airway intervention.
When assessing stridor, the location of the turbulent airflow may be determined by the respiratory phase in which the sound is noted. There are three zones of the airway that are responsible for these sounds- supraglottic, glottic and subglottic, and intrathoracic. Supraglottic obstruction results in a high-pitched, inspiratory stridor. Obstruction of the extrathoracic trachea, including the glottis and subglottis, is characterized by biphasic stridor with an intermediate pitch. Obstruction of the intrathoracic trachea (including first and second order bronchi) results in expiratory stridor, or wheezing. This last area of obstruction is associated with retraction of the sternum, costal cartilage, and suprasternal tissue.
The differential diagnosis of noisy breathing in children can be grouped into the anatomic location of the lesion or defect. Underlying causes can be classified as congenital, inflammatory, neoplastic, neuromuscular, and traumatic. The following is a list of diagnoses depending on the location of the lesion or defect:
Nose and nasopharynx-
- Congenital: choanal atresia or stenosis, pyriform aperture stenosis, craniofacial anomalies
- Inflammatory: nasal polyps, rhinitis, retropharyngeal abscess, adenoid hypertrophy
- Neoplastic: encephalocele, dermoid, glioma
- Traumatic: foreign body
- Oropharynx/hypopharynx-
- Congenital: glossoptosis/macroglossia, lingual thyroid, vallecular cyst, craniofacial anomalies
- Inflammatory: tonsil hypertrophy, retropharyngeal abscess
- Neoplastic: dermoid, hemangioma, lymphangioma
- Neuromuscular: hypotonia, neurologic disease
- Traumatic: foreign body
Supraglottic larynx-
- Congenital: laryngomalacia, laryngocele/saccular cyst
- Inflammatory: epiglottitis, angioneurotic edema
- Neoplastic: hemangioma, lymphangioma, papilloma
- Traumatic: foreign body
Glottic larynx-
- Congenital: web/atresia, laryngeal cleft, stenosis
- Inflammatory: laryngitis, spasm, stenosis
- Neoplastic: hemangioma, lymphangioma, papilloma, granuloma
- Neuromuscular: vocal cord paralysis
- Traumatic: hematoma, fracture, foreign body, stenosis
Subglottic larynx-
- Congenital: stenosis, cysts
- Inflammatory: croup, stenosis
- Neoplastic: hemangioma, papilloma
- Traumatic: chondritis, stenosis, fracture, foreign body
Tracheobronchial-
- Congenital: stenosis/web, tracheomalacia, vascular ring/sling/complete tracheal rings, foregut cysts, tracheoesophageal fistula
- Inflammatory: membranous tracheitis, bronchitis, asthma
- Neoplastic: mediastinal tumors, thyroid, thymus, papilloma
- Traumatic: stenosis, foreign body (tracheal or esophageal)
Laryngomalacia is the most common cause of stridor in infancy. Furthermore, it is the most common congenital laryngeal anomaly. Males are affected twice as often as females.
Laryngomalacia arises from a continued immaturity of the larynx. Symptoms of laryngomalacia are often not present at birth. In fact, onset of symptoms typically occurs days to weeks after birth (most commonly within the first two weeks of life) and they will resolve at twelve to eighteen months of age without intervention. The diagnosis is suspected when inspiratory stridor is auscultated and is confirmed by flexible nasopharyngoscopy.
Typical stridor associated with laryngomalacia is low in pitch with a fluttering quality secondary to the circumferential rimming of the supraglottic airway and aryepiglottic folds. It is often associated with general noisy respiration. The stridor is most prominent when the child is in the supine position or when the child is agitated. More forceable inspiration tends to result in a louder stridor quality due to greater prolapse and thus greater obstruction.
Radiographic studies can suggest the diagnosis of laryngomalacia, however the mainstay of diagnosis is flexible nasopharyngoscopy. For completeness, we will include radiographic findings. An inspiratory film with neck extension can show medial and inferiorly displaced arytenoids and epiglottis. Fluoroscopy can demonstrate the collapse of the supraglottic structures with inspiration. Chest radiographs can aid in evaluation of potential lower respiratory tract anomalies such as innominate artery compression, tracheomalacia, and vascular rings.
Flexible nasopharyngoscopy is best performed in an unanesthetized child in an upright position with a 1.9mm nasopharyngoscope. The scope should be passed through both nasal passages when examining the patient. Classic findings with flexible nasopharyngoscopy are a cyclical collapse of the supraglottic larynx with inspiration. The aryepiglottic folds are short and often times there is an omega shaped epiglottis. Short aryepiglottic folds draw the cuneiform and corniculate cartilages forward over the laryngeal inlet resulting in prolapse during inspiration. These findings will be reviewed when discussing the five types of laryngomalacia. It should be noted that the vocal cords are mobile in laryngomalacia. Although laryngomalacia can often make visualization of the vocal cords difficult, they still must be examined.
Multiple factors may contribute to the development of laryngomalacia. The main contributors are thought to be anatomic, neurologic, and inflammatory. The underlying anatomic pathologies are shortening of the aryepiglottic folds and anterior collapse of the cuneiform cartilages. Immature neuromuscular control and movement has also been thought to impact laryngomalacia. Additionally, there is an association of gastroesophageal reflux and laryngomalacia. Reflux can induce posterior supraglottic edema and secondarily laryngomalacia.
A recent article published in April 2005 by Dr. SC Manning et al. evaluated the anatomical impact on laryngomalacia. The specific objective of the article was to compare the aryepiglottic length in pediatric patients who have severe laryngomalacia and are undergoing aryepiglottoplasty with the aryepiglottic length of a sample of control patients without laryngomalacia. The study design was a prospective case-control. Measurements were compared by creating a ratio of the aryepiglottic fold length (distance from the most anterior arytenoids cartilage to the closest posterior lateral edge of the epiglottic cartilage) to the glottic length (distance from the anterior border of the interarytenoid muscle to the anterior commissure). The mean ratio of patients with severe laryngomalacia was calculated to be 0.380, while the mean ratio of control patients was calculated to be 0.535.
There are five types of laryngomalacia. Type I is an inward collapse of the aryepiglottic folds (mainly the cuneiform and corniculate cartilages). These cartilages are drawn inward during inspiration and open passively during expiration. Type II is a long, tubular epiglottis which curls on itself and contributes to obstruction during inspiration. This often occurs in association with Type I laryngomalacia. Type III is anterior, medial collapse of the cuneiform and corniculate cartilages to occlude the laryngeal inlet during inspiration. Type IV is posterior inspiratory displacement of the epiglottis against the posterior pharyngeal wall or inferior collapse to the vocal folds. Type V is short aryepiglottic folds.
The requirement of surgical intervention in patients with laryngomalacia is rare, as it is generally a self-limiting condition. Severe symptoms such as inability to feed orally, cor pulmonale, failure to thrive, and life threatening airway obstruction may necessitate surgical intervention. Prior to the 1980s, tracheotomy was used in this setting. The rationale for this intervention was that a tracheotomy bypassed the area of obstruction until the supraglottic pathology spontaneously resolved. Today, this strategy is only employed in the severely affected infant. Instead, the strategy has changed to address the area of obstruction directly with a supraglottoplasty. This can be performed by making use of microlaryngeal instruments, a carbon dioxide laser, or a microdebrider to excise redundant mucosa and/or cuneiform cartilages as well as releasing the shortened aryepiglottic folds and trimming the lateral edges of the epiglottis. It is important to note that there is more than one obstructing mechanism in laryngomalacia and
when performing supraglottoplasty, all potential mechanisms should be addressed. When removing tissue, the surgeon should be conservative, as supraglottic stenosis can result from excessive tissue removal. Unilateral supraglottoplasty should be considered and the second side should be operated on only if symptoms do not resolve. Also, direct laryngoscopy and bronchoscopy must be performed prior to supraglottoplasty to rule out concomitant pathology contributing to the airway obstruction. The direct laryngoscopy should be performed when the patient is breathing spontaneously under general anesthesia. Post-operatively, the patients are usually left intubated over night with extubation the following morning. Antibiotics should be given at least five days post-operatively to prevent infection. Antireflux precautions including both positioning and medication are recommended to minimize the raw mucosal surfaces exposure to gastric secretions. Below is a review of the literature
The original description of endoscopic removal of supraglottic tissue with a nasal snare for laryngomalacia was first described in 1922 by Dr. S Iglauer. In 1984, Dr. RW Lane et al. described a single report of the removal of the corniculate cartilages and redundant arytenoids mucosa in a child with laryngomalacia. In 1985, Dr. AB Seid et al. described the use of the carbon dioxide laser for treatment of laryngomalacia in a series of three patients.
In 1987, GH Zalzal et al. presented epiglottoplasty as a new procedure. His data included a case series of ten patients. He described a laryngoscopic approach in which redundant mucosa was excised from the lateral edges of the epiglottis, aryepiglottic folds, arytenoids, and corniculate cartilages. This was done with the patient in the supine position while visualizing the larynx through a laryngoscope. The tissue to be excised was grasped with cup forceps and trimmed with Bellucci scissors. Dr. Zalzal reported that “all patients who underwent epiglottoplasty achieved complete relief from their symptoms with the exception of one patient in whom mucosa from the aryepiglottic folds and arytenoids area had not been adequately excised. Further excision resulted in complete relief.” His indications for operating on a patient with laryngomalacia were severe stridor not resolved with time that may be associated with failure to thrive, cor pulmonale, feeding difficulties, and apnea. Additionally, inability to view the vocal cords with a flexible nasopharyngoscope during inspiration because of laryngeal inlet collapse is a clear indication for surgery.
In 1995, G Roger, et al. published a retrospective study of 115 cases of resection of the aryepiglottic folds with or without a carbon dioxide laser. The success rate was determined to be 98% with an average follow-up period of thirty months. Failure of the procedure was assigned to two children who needed tracheotomies. Seven patients required a revision surgery. In this paper, Dr. Roger established criteria for the definition of severe laryngomalacia:
1. Dyspnea at rest and/or severe dyspnea during effort
2. Feeding difficulties
3. Height and weight growth rate stagnation
4. Sleep apnea or obstructive hypoventilation
5. Uncontrollable gastroesophageal reflux
6. History of intubation for obstructive dyspnea
7. Effort hypoxia (10% higher than the normal values for the same age group)
8. Effort hypercapnia (10% higher than the normal values for the same age group)
9. Abnormal polysomnography with an increased apnea/obstructive hypoventilation index
The presence of at least three of these criteria was deemed to be a formal indication for endoscopic surgery.
Dr. SM Kelly et al. evaluated the effectiveness of unilateral endoscopic supraglottoplasty for treatment of severe laryngomalacia in 1995. The study design was a retrospective review of eighteen patients with severe laryngomalacia that had undergone unilateral carbon dioxide laser removal of redundant supraglottic tissue. Three of the eighteen patients required a contralateral supraglottoplasty. Relief of obstructive symptoms was achieved in 94% of the patients. The one patient without obstructive relief had tracheomalacia secondary to previous tracheotomy for severe laryngomalacia.
In 1999, DR Olney et al. performed a retrospective chart review aimed at determining the airway outcome of infants with laryngomalacia who do not undergo routine direct laryngoscopy and bronchoscopy, the age at which laryngomalacia resolves, and the outcome of supraglottoplasty as a function of the type of laryngomalacia and the presence of concomitant disease. Dr. Olney found that direct laryngoscopy and bronchoscopy as part of the routine evaluation of laryngomalacia is not warranted and should only be performed when there is clinical and physical evidence of a concomitant airway lesion. The median time to resolution of isolated laryngomalacia was 36 weeks, and by 72 weeks, 75% of infants were free of stridor. This finding did not vary significantly in infants with severe neurological compromise or in infants with other congenital anomalies. Supraglottoplasty was determined to be necessary in approximately 15-20% of affected infants which is attributed to episodes of apnea and failure to thrive.
A chart review was performed in 2001 by CW Senders et al. to evaluate different carbon dioxide laser procedures on children with various types of laryngomalacia and determine the role of associated anomalies on the outcome. Twenty-three children were included in a retrospective chart review that underwent carbon dioxide laser vaporization of redundant supraglottic mucosa of the aryepiglottic fold, arytenoids, and the epiglottis. Patients without associated anomalies did well, with 78% immediately resolving their respiratory symptoms and 100% resolving their respiratory symptoms within a week. Unfavorable immediate results, as well as long term surgical failures all had associated anomalies including Arnold-Chiari, Cerebral Palsy, CHARGE association, and Rieger syndrome. Conclusions determined by Dr. Senders were that laser supraglottoplasty is a safe and effective treatment for all types of laryngomalacia, but children with associated neurological or anatomic anomalies will have a more complicated immediate and short term course, as well as a significant incidence of failure.
A retrospective review was performed in 2001 by SC Toynton et al. looking at one hundred patients that had endoscopic aryepiglottoplasty performed for severe laryngomalacia. Surgery was used for treatment of patients that had oxygen saturation below 92% and feeding difficulties causing failure to thrive. Ninety-four percent of the patients had improvement of their stridor after one month, with 55% completely without stridor. Patients with slower progression of improvement were found to have a serious neurological condition. Seventy-two percent of patients with preoperative feeding difficulties improved their feeding. Dr. Toynton concluded that endoscopic aryepiglottoplasty is the operation of choice for severe laryngomalacia. However, underlying neurological disease decreases the success rate of operative intervention.
In 2001, Dr. DK Reddy et al. performed a retrospective review to evaluate the efficacy of unilateral supraglottoplasty compared to bilateral supraglottoplasty. This study included 106 patients- 59 patients undergoing bilateral supraglottoplasty and 47 patients undergoing unilateral supraglottoplasty as the initial surgery. Surgical success was defined as the resolution of clinically significant laryngomalacia and was reported to approach 96% in this study. Fifteen percent of the patients that had unilateral supraglottoplasty performed needed an additional contralateral procedure performed for resolution of symptoms. Two patients (3%) that underwent bilateral supraglottoplasty initially developed supraglottic stenosis. No patients undergoing unilateral supraglottoplasty developed supraglottic stenosis.
Dr. Reddy recommended that unilateral supraglottoplasty is a reasonable initial surgical management of pediatric patients with severe laryngomalacia.
Dr. D Loke et al. performed a retrospective review in 2001 that examined the outcome of 32 cases of severe laryngomalacia that underwent a simple division of the aryepiglottic folds. Approximately 69% showed complete resolution of stridor and associated complications of laryngomalacia, while 22% showed partial resolution of stridor with no further surgical intervention required. Six percent required one additional procedure which included more extensive excision of redundant mucosa. One patient required a tracheotomy. It was concluded that endoscopic excision of the aryepiglottic folds is highly effective and should be the first-line treatment for severe laryngomalacia.
In 2002, isolated posterior displacement of the epiglottis was addressed by Dr. JA Werner et al. by performing an epiglottopexy for the treatment of severe laryngomalacia. Six patients underwent epiglottopexy- 4 of which were solely epiglottopexy and 2 of which were combined epiglottopexy with transaction of the aryepiglottic folds. The procedure was performed transorally with a carbon dioxide laser. All six children demonstrated significant airway improvement without any further stridor. It was also noted that deglutition was not affected by this procedure.
The failures and complications of supraglottoplasty were analyzed in 2003 by Dr. F Denoyelle et al. The study design was a retrospective review that included 136 patients, 102 of which had isolated laryngomalacia and 34 of which had additional congenital anomalies (Pierre Robin, psychomotor retardation, CHARGE association, Down syndrome, miscellaneous). Outcome measures included persistence of dyspnea, sleep apnea, and/or failure to thrive; need for additional treatment; presence of granuloma, edema or web; and supraglottic stenosis. Failure or only partial improvement of symptoms was only seen in patients with additional congenital anomalies (8.8%). The need for revision surgery was 4.4% of patients, minor complications (granuloma, edema or web) occurred in 3.7% of patients and supraglottic stenosis occurred in 4.4% of patients. Dr. Denoyelle concluded that supraglottoplasty failed only in patients that had laryngomalacia and additional congenital anomalies. The complication rate was found to be similar between isolated laryngomalacia and patients that had laryngomalacia with additional congenital anomalies.
This year (2005), Dr. GH Zalzal et al. presented a new approach to supraglottoplasty by making use of the microdebrider. He reported on a series of five patients that had been diagnosed with sever laryngomalacia and underwent a microdebrider-assisted supraglottoplasty. The technique involved initially dividing the aryepiglottic fold with microlaryngeal scissors. Subsequently, the folds are resected with the microdebrider with tissue removal extended anteriorly to the lateral edge of the epiglottis and posteriorly to the arytenoids cartilage. If redundant supra-arytenoid mucosa was identified, it was removed with the microdebrider. All five patients had resolution of their stridor postoperatively. No complications including supraglottic stenosis have been identified and no revision surgeries have been performed.
When evaluating an infant that presents with stridor, laryngomalacia tops the differential diagnosis. It is established that the majority of symptoms of laryngomalacia resolve without intervention by the ages of twelve to eighteen months. The otolaryngologist must decide if the associated symptoms justify operative intervention. Furthermore, it must be decided if there are associated congenital anomalies that could impact the success of the surgery. If surgical intervention is pursued, all potential mechanisms contributing to laryngomalacia should be addressed.
SUBGLOTTIC STENOSIS
Introduction
Subglottic stenosis (SGS) is a congenital or acquired narrowing of the subglottic airway. In the early twentieth century SGS was rare. Most cases occurred in adults. In the 1960s the incidence of acquired SGS began to dramatically increase in the neonatal population. This resulted from the increased survival of low-birth-weight infants and the increased use of intubation in this population. In addition, long term intubation became accepted as an alternative to tracheotomy. As a consequence the management of this condition underwent a revolution in the 1970s and reconstructive surgery was directed towards this population.
Management of laryngotracheal stenosis (LTS) is one of the most challenging problems for the head and neck surgeon to be faced with. Most of these patients are referred to and are treated at large academic centers by physicians specialty trained in this area. Particularly in the pediatric population, a multidisciplinary approach is taken to manage this complex problem.
Anatomy
The subglottis is defined as the area extending from the lower surface of the true vocal cords to the lower surface of the cricoid cartilage. In adults this corresponds to approximately 10 mm inferior to the anterior commissure and 5 mm inferior to the posterior commissure.
The infant larynx differs significantly in size and position when compared to the adult larynx. At birth, the infant larynx is approximately one third the size of the adult larynx, however, the infant larynx is proportionately larger than the adult larynx compared with the remainder of the tracheobronchial system. The vocal process of the arytenoid takes up half the length of the vocal cord in the infant larynx, while it only takes up about ¼ of the length of the vocal cord in the adult. The narrowest portion of the airway in the older child and adult is the glottic aperture, while the narrowest part of the airway in the infant is the subglottis. The subglottis in infants measures approximately 4.5 by 7mm. A diameter of 4.0 mm is considered the lower limit of normal in a full term infant and 3.5 mm in a premature infant. One millimeter of edema circumferentially in the subglottis reduces the cross-sectional area by 60%.
The infant larynx is positioned higher in the neck than the adult larynx. The superior border of the larynx of the infant is located at about the level of the first cervical vertebrae with the cricoid positioned at about the fourth cervical vertebrae. In comparison, the adult cricoid rests at about the level of the sixth cervical vertebrae. The structures of the infant larynx are more pliable and less fibrous making the infant airway more susceptible to narrowing from edema and less easily palpable.
Embryology
The respiratory system is an outgrowth of the primitive pharynx. The development of the lower respiratory system begins at 26 days after conception as the laryngotracheal groove at the ventral aspect of the foregut forms.
The laryngotracheal diverticulum becomes separated from the foregut by the tracheoesophageal folds which fuse to become the tracheoesophageal septum. This septum divides the foregut into a ventral laryngotracheal tube and a dorsal esophagus. Failure of the tracheoesophageal folds to fuse during the fourth and fifth weeks can lead to a tracheoesophageal fistula.
The larynx develops from the fourth and fifth branchial arches. The laryngotracheal opening lies between these two arches. This primitive laryngeal aditus is altered to become a T-shaped opening by the growth of three tissue masses. One is the hypobranchial eminence. This mesodermal structure eventually becomes the epiglottis. The second and third growths are two arytenoid masses. As these masses grow between the fifth and seventh weeks, the laryngeal lumen is obliterated. Recanalization occurs by the tenth week. Failure to recanalize may result in atresia, stenosis, or web formation in the larynx. The arytenoid masses are separated by an interarytenoid notch which eventually becomes obliterated. If obliteration does not occur, a posterior laryngeal cleft can result leading to severe aspiration in the newborn
Etiology of SGS
I. Congenital SGS
A. Membranous
- increased fibrous connective tissue
- hyperplastic submucous glands
- granulation tissue
B. Cartilagenous
- cricoid cartilage deformity
a. small cricoid
b. elliptical cricoid
c. large anterior lamina
d. large posterior lamina
e. generalized thickening
f. submucous cleft
2. trapped first tracheal ring
II. Acquired SGS
A. Intubation
B. Laryngeal trauma
a. previous airway surgery
– high tracheostomy
– cricothyroidotomy
– prior surgery for respiratory papillomatosis
– prior laser surgery for SGS
b. accidental
1. inhalational (thermal or caustic)
2. trauma (blunt or penetrating)
C. Autoimmune
D. Infection
E. Gastroesophageal reflux (GER)
F. Inflammatory diseases
a. Anti-neutrophil Cytoplasmic Autoantibodies (C-ANCA)
b. sarcoidosis
c. Systemic lupus erythematosis
G. Neoplasms
III. Idiopathic SGS
Congenital SGS
SGS may be classified as either acquired or congenital. Although congenital subglottic stenosis is uncommon, accounting for 5% of all cases, it is the third most common congenital airway problem (after laryngomalacia and vocal cord paralysis). Congenital SGS is thought to be secondary to failure of the laryngeal lumen to recanalize properly during embryogenesis. SGS is considered congenital if there is no history of endotracheal intubation or other forms of laryngeal trauma.
Congenital SGS is divided histopathologically into membranous and cartilaginous types.
Membranous SGS is usually circumferential and consists of fibrous soft-tissue thickening caused by increased fibrous connective tissue or hyperplastic submucous glands. It may involve the vocal folds as well. The cartilaginous type usually results from a thickened or deformed cricoid cartilage that forms an anterior subglottic shelf that extends posteriorly allowing only a small posterior opening. Other malformations can occur such as an elliptical cricoid leaving a slit-like opening or a trapped first tracheal ring. Membranous SGS is usually less severe than the cartilaginous type.
Congenital SGS is often associated with other congenital malformations. A thorough search for associated anomalies is necessary.
Acquired SGS
Ninety-five percent of cases of SGS are acquired and may be due to a number of causes. The most common cause of acquired SGS (90%) results from endotracheal tube intubation and the associated inflammatory-type response (see below – pathology). The most important risk factor for the development of laryngotracheal stenosis is the duration of intubation. Other factors include size of the endotracheal tube, movement of the endotracheal tube, traumatic intubation, number of re-intubations, and presence of an infection while intubated.
Other causes of acquired SGS include external and post-surgical trauma, and systemic factors. Laryngotracheal injuries from blunt trauma may result in fractures, dislocations, mucosal lacerations, or cricotracheal separation. Wound healing in these instances occurs as described below with development of granulation tissue and then deposition of fibrous tissue and subsequent scarring which may result in clinically apparent stenosis. Caustic and thermal injuries also cause mucosal injury, ischemia and subsequent remodeling of tissues and may result in LTS.
Post-surgical trauma includes previous tracheotomy (particularly high tracheotomy), cricothyroidotomy, and surgical treatment of airway neoplasms (most commonly recurrent respiratory papillomas and subglottic hemangiomas). The incidence of subglottic stenosis has been reported to be as high as 20% in patient undergoing laser excision of subglottic hemangiomas (Sie, 1994).
Gastroesophageal reflux (GER) has been proposed as a medical condition which may exacerbate the pathogenesis of LTS, may cause re-stenosis after repair, and may be the sole cause of stenosis in patients with no previous history of endotracheal intubation or laryngotracheal trauma. Bain et al. (1983) was the first to suggest that GER may cause SGS. He identified GER in two patients with SGS. Since then animal studies have been performed which have shown that superficial mucosal injury in the trachea and subglottis treated with acid and acid with pepsin result in non healing and inflammation (Koufman, 1991) and may lead to SGS (Little, 1985). Little’s study, however, had only one subject in each group and therefore significant results were not obtained. Other studies have shown a high rate of GER in patients with SGS. Walner et al. (1998) restropectively looked at 74 pediatric patients with SGS and found that they had a three times greater incidence of GER than the general pediatric population. They were unable to draw any conclusions about the relationship between GER and SGS because of the diversity of their patient group. Koufman (1991) found that 72% of 32 patients (11 pediatric and 21 adult) with laryngeal and tracheal stenosis had abnormal lower pH probe results. 67% of those patients had abnormal pharyngeal reflux defined as a single episode of a pH less than 4.0.
The current criteria for diagnosing lower esophageal acid reflux is well documented (a pH of less than 4.0 for more than 10% of the time), however, despite the fact that pH-metry is considered the gold standard for LPR testing, there is no consensus with respect to the number of pH sensors, their location, or the interpretation of results in adults or children (Postma, 2002). Postma suggests four criteria that must be met for an event to be defined as a pharyngeal reflux episode:
(1) A decrease in the pH level to less than 4.0 (may be increased to 5.0)
(2) A decrease in the pharyngeal pH level immediately following distal esophageal acid exposure
(3) No decrease in the pH level during eating or swallowing
(4) A rapid and sharp decrease in the proximal sensor pH level rather than a gradual one
Pepsin is functional at a pH of 5.0. The laryngopharynx is much more susceptible to the inflammatory affects of acid and pepsin because it lacks the protective mechanisms that the stomach and lower esophagus have. As was shown in Koufman’s study (1991), nonhealing ulcers and inflammation resulted in areas of damage to the subglottis as a result of acid and pepsin administration. It is logical to think that exposure to a single episode of LPR in patients who have existing laryngeal or subglottic injury may have significant effects.
Despite the lack of definitive evidence for a cause and effect relationship between GER and SGS, Cotton and O’Connor state that a “reflux workup” is now considered essential to the success of laryngotracheal reconstruction (LTR) (1995). Empirical treatment of GER has been recommended for all patients undergoing LTR (Burton, 1997) even if they do not have symptoms of GER or LPR or documented reflux by pH probe.
Idiopathic subglottic stenosis
The pathogenesis of acquired subglottic stenosis is not completely understood but there are several theories that have been proposed. The most accepted theory proposes that subglottic stenosis results from wound healing in the areas of the airway which have undergone compression by an endotracheal tube or the cuff of a tube resulting in necrosis of the underlying mucosa and cartilage. This necrosis is a consequence of ischemia resulting from pressure from the tube or cuff exceeding the capillary pressure of the thin mucosa of the airway. Consequently, the normal mucociliary flow is disrupted which leads to infection in the perichondrium and then extends into cartilage. The cartilage may weaken and collapse, manifesting as tracheomalacia. Healing of the involved segment proceeds by secondary intention. This involves three temporally overlapping stages: an inflammatory stage, a proliferative stage, and a phase of contraction and remodeling. The inflammatory stage begins with the initial injury. It involves active vascular retraction and constriction followed by vasodilation mediated by prostaglandins. Platelets adhere to the exposed collagen forming a hemostatic plug. There are numerous active mediators that are released including adenosine diphosphate, platelet derived growth factor, transforming growth factor, clotting factors and vasoactive substances such as 5-hydroxytryptamine, thromboxane and histamine. The coagulation cascade is activated which lays down the fibrin network. Platelets release proteolytic enzymes that activate the complement system. The complement cascade contributes to bacterial killing and wound debridement and supplies chemotactic signals to effect cells.
Polymorphonuclear lymphocytes (PMNs) enter the wound at about 6 hrs post-injury. They reach the their greatest concentration at 24-48hrs post-injury and disappear within 72hrs. The PMNs phagocytize debris and bacteria from the wound and release proteases that lyse devitalized tissue.
T and B cells enter the wound after the PMNs and are most numerous at 6 days after injury. T cells secrete regulatory lymphokines. Helper T-cells are necessary for wound healing. Suppressor T cells down-regulate healing.
Macrophages enter the wound within 48-96 hrs after injury. Large concentrations of macrophages are found in the wound at 3-5 days and can persist for several weeks. Macrophages are the only cells that can function in area of low oxygen tension at the wound center. These cells release many different enzymes but they primarily direct the proliferative and synthetic activity in the wound. This is achieved by interleukin-1 and tumor necrosis factor –alpha. Studies have shown that macrophages are essential to wound healing but PMNs can be absent without impairing the process.
Growth factors secreted by cells in the wound are major regulators of healing. Growth factors are chemotactic, mitogenic, and sometimes have an inhibitory effect on cells involved with wound repair. All growth factors interact with cellular receptors to modify the cells activities. IL-1 directly stimulates fibroblast activity including proliferation and collagen synthesis. IL-2 is produced by helper T cells. It has an indirect effect on wound healing by stimulating lymphocyte and macrophage activity.
Structural components essential to successful wound healing include fibronectin, collagens, glycoproteins and glycosaminoglycans. Fibronectin is secreted by fibroblasts and endothelial cells. It cross-links fibrin and glycosaminoglycans. Several types of collagen are found in the healing wound. The production of collagen by fibroblasts is a complex process. Hydroxylation requires Vitamin B, iron and Vitamin C. Glycoproteins and glycosaminoglycans function in cell-matrix interactions and in controlling wound resilience.
The proliferative phase lasts 10-14 days. It begins with re-epithelization. This process of resurfacing the defect begins at about 12 hrs after injury. The epithelial cells 1-2 mm from the wound edge undergo phenotypic changes. The cells replication rate increase 17 fold.
The next part of the proliferative phase is neovascularization in which blood vessel regeneration begins with the migration of endothelial cells. Macrophages secrete angiogenic factors in response to high lactate levels and low wound oxygen levels. The result of endothelial migration is capillary bud formation. Certain conditions such as diabetes mellitus or radiation therapy impede neovascularization.
Collagen deposition begins when fibroblasts enter the wound at 48-72 hrs. post-injury. The collection of fibroblasts, inflammatory cells and capillary buds is referred to as granulation tissue. Granulation tissue persists until epithelial resurfacing is complete.
The final phase of wound healing is the wound contraction and remodeling phase. Wound contraction begins at 6-7 days after injury and is maximal for 10 days. Wound contraction eventually decreases the defect by 40-60%. Skin flaps and grafts can reduce the amount of wound contraction by 50-70%. Myofibroblasts, a modified fibroblast, provides the contractile force required for contraction. They have many characteristics of smooth muscle cells and are distributed throughout the wound. Wound collagen levels reach maximum at 2-3 weeks after injury, however, tissue strength is still only 5-10% of that of unwounded skin. The wound’s neo-matrix is gradually replaced over 6-12 months by stronger interwoven cartilage as Type I collagen displaces Type III cartilage. Remodeling results in a scar with as much as 80% of the involved tissue’s original tensile strength.
Diagnosis
There is a wide range of presentation of subglottic stenosis with similarities and differences in the pediatric age group compared to adults. If the stenosis is severe and congenital, the patient will present with airway distress at birth. More commonly, the pediatric patient with SGS with present as a neonate in the intensive care unit who has failed extubation usually multiple times. Occasionally patients will present with in clinic with a tracheotomy and the report of some airway obstruction. Infants with mild SGS may present with recurrent croup-like illnesses and poor feeding. Adults usually present with a history of prior intubation with symptoms of progressive shortness of breath and noising breathing.
In the infant or child, a thorough history should be obtained which includes: history of prematurity and associated medical problems and intubation records. A history of noisy breathing and difficulty feeding should lead to suspicion of airway problems. Growth curves should be reviewed and followed to determine if the child has failure to thrive. Particularly the relationship of airway symptoms to feeding is important to elicit in the history. Important characteristics of the intubation include the date of first intubation, duration, size of the endotracheal tube, number of intubations, and if any intubations were traumatic.
In adults, intubation records should be reviewed as well. A complete medical history is taken with particular attention to the patients cardiopulmonary status, history of diabetes, and any steroid use. History of reflux symptoms should be elicited which may prompt subsequent testing.
Differential diagnosis of laryngotracheal stenosis
I. Congenital
A. Tracheomalacia
B. Laryngomalacia
C. Vocal cord paralysis
D. Laryngeal cleft
E. Congenital cysts
D. External compression from congenital abnormality or lesion
1. Vascular compression
a. innominate artery compression (most common)
b. right-sided aortic arch with persistent ductus arteriosus
c. aberrant left pulmonary atery
2. Mass
a. teratoma
b. cystic hygroma
c. hemangioma
II. Infectious/inflammatory
A. Viral laryngotracheobronchitis (croup)
B. Retropharyngeal abscess
C. GER
D. Tracheitis
III. Neoplastic
A. Subglottic hemangioma
B. Recurrent respiratory papillomatosis
IV. Traumatic
External compression
Foreign body
A complete head and neck exam should be performed on all patients. It begins with observing the patient for any apparent airway symptoms which may include irritability and restlessness in an infant or dyspnea, tachypnea, cyanosis, and stridor in an infant, child, or adult. The classic stridor of subglottic stenosis is biphasic. Voice quality in a child or adult and crying quality in an infant should be evaluated for weakness, hoarseness, breathiness, or complete absence. Flexible fiberoptic nasopharyngolaryngoscopy may be performed at the bedside or in clinic on infants, adults, and some cooperative children if the patient is stable. Vocal cord immobility, reflux changes, immediate subglottic abnormalities, supraglottic sensation, and other glottic and supraglottic abnormalities may be detected.
The gold standard for diagnosis of any laryngotracheal abnormalities is direct laryngoscopy and tracheobronchoscopy under general anesthesia. This should be performed in the operating room with an experienced anesthesiologist. It is important to delay endoscopy for at least two weeks following an acute episode of croup to minimize the risk of postoperative airway obstruction. The potential need for tracheotomy should be discussed with the patient (adults) or patient’s family (children) prior to endoscopy.
A rigid bronchoscope or a rod lens telescope may be used to assess the airway. The important things to document during endoscopy are as follows: (1) the outer diameter of the largest bronchoscope or endotracheal tube that can be passed through the stenotic segment, (2) the location/subsites (glottis, subglottis, trachea) and length of the stenosis, (3) other separate sites of stenosis, (4) other airway anomalies in infants (clefts, webs, cricoarytenoid joint fixation, neoplasms, etc.), and (5) reflux changes. After removing the sizing endotracheal tube or bronchoscope it is important to observe the stenotic segment for edema which may result in the need for tracheostomy.
There are two widely excepted staging systems for classifying subglottic stenosis: Myer-Cotton grading system and the McCaffrey system. Other systems have been described as well, however, none are universally applicable or useful. At this time, no staging system exists that allows comparison of patients treated at different institutions.
The Myer-Cotton staging system is useful for mature, firm, circumferential stenosis confined to the subglottis. It describes the stenosis based on the percent relative reduction in cross-sectional area of the subglottis which is determined by differing sized endotracheal tubes.
Four grades of stenosis are described with this system: grade I lesions have less than 50% obstruction, grade II lesions have 51% to 70% obstruction, grade III lesions have 71% to 99% obstruction, and grade IV lesions have no detectable lumen or complete stenosis.
The McCaffrey system classifies laryngotracheal stenosis based on the subsites involved and the length of the stenosis. Four stages are described: stage I lesions are confined to the subglottis or trachea and are less than 1cm long, stage II lesions are isolated to the subglottis and are greater then 1 cm long, stage III are subglottic/tracheal lesions not involving the glottis, and stage IV lesions involve the glottis.
Another classification system has been proposed by Lano et al. at Vanderbilt University (1998) for adults. There system is based on the number of subsites (including the glottis, subglottis and trachea) of involvement: stage I lesions involve one subsite, stage II involves two subsites and stage III involves all three subsites. The authors proposed this classification to better predict patient prognosis (successful decannulation) after reviewing their experience over ten years.
Radiographic tests that may be ordered to evaluate the patient with upper airway problems include plain films, barium esophagram, airway fluoroscopy, magnetic resonance imaging (MRI), and computed tomography (CT). Plain films are more useful in the pediatric population and may be used to diagnosis foreign bodies, narrowing of the subglottis and trachea in croup, epiglottis, retropharyngeal abscess, subglottic cystes, and subglottic hemangioma. They are cheap, easily accessible, and can be done without sedation. If adequate inspiratory and expiratory films cannot be obtained, fluoroscopy may be used. Airway fluoroscopy is dynamic and can show areas of malacia. Barium swallow studies are not useful specifically in the diagnosis of SGS but are indicated when assessing feeding problems in infants and can be used to rule out other anatomic abnormalities such as esophageal compression from vascular anomalies, esophageal masses and may show evidence of reflux. MRI is useful in diagnosing soft tissue masses. CT scanning with thin cuts (1.5) through a stenotic segment of the trachea and/or subglottis can help determine the length of resection that will be needed. CT has not been shown to be useful in diagnosing or characterizing SGS in the pediatric age group but is useful in adults as an adjunct to endoscopy. MRI and CT usually require sedation or general anesthesia in young children and infants.
Management
As previously mentioned, all patients must undergo a complete history, physical examination, flexible nasopharyngolaryngoscopy and +/- radiologic evaluation to make the diagnosis. This is followed by rigid endoscopy to evaluate the larynx, trachea, bronchi, and esophagus and to confirm the diagnosis and characterize the stenotic lesion. The management of the lesion will then depend on its diameter, length, location and the status of the patient.
Medical
Prior to airway reconstruction, it is recommended that all pediatric patients be evaluated for GER with a dual 24hr pH probe(Cotton and Walner, 1999). This is performed with the consultation of a pediatric gastroenterologist. Different sized pH probes are used depending on the age of the child. Position of the probe should be confirmed with fluoroscopy. In unusual cases the refluxate may be basic in pH. In these patients, diagnosis is made with a nuclear medicine reflux scan. Patients diagnosed with GER should be treated accordingly. Adults are not always subjected to an extensive workup for GER unless symptoms are present. Empiric peri-operative treatment with anti-reflux medications has been recommended by some authors and is practiced by many.
In adults it is important to evaluate the patients general medical condition prior to performing any reconstructive procedures. Many of these patients underwent long periods of intubation secondary to severe medical problems. The decision to perform surgery should be made in consultation with the patient’s primary or specialty physician (pulmonary, cardiology, nephrology, etc.). Relative contraindications to LTR in adults are renal failure, diabetes, severe coronary artery disease, severe COPD or restrictive lung disease, obstructive sleep apnea, and systemic steroid use. It is important to consider each patient’s case on an individual basis and make the decision to proceed with surgery based on sound judgment.
Observation
Patients (children and adults) with Cotton-Myer grade I and mild grad II subglottic stenosis may sometimes be managed with close observation (Walner and Cotton,1999). In adults, this will depend on the reliability of the patient for close follow-up and their symptomatology. Children may be watched closely if they have only occasional mild stridor without retractions or feeding difficulties and have not required hospitalization for episodes of croup or other airway-related illnesses. Walner and Cotton recommend repeat endoscopy every three to six months to measure the diameter of the airway to ensure that it is enlarging as the child grows. As stated previously, the child should be followed with growth curves by a pediatrician and/or neonatologist.
Surgical treatment options for subglottic stenosis:
I. Tracheostomy
II. Endoscopic
A. Dilation
B. Endoscopic laser excision
III. Open procedure
A. Expansion procedure (one-stage or with stent placement)
1. Anterior cricoid split with or without cartilage graft*
2. Posterior cricoid split with or without cartilage graft*
3. Anterior and posterior cricoid split with cartilage graft
4. Four quadrant LTR
B. Segmental resection (cricotracheal resection – CTR)
1. Primary CTR
2. Salvage CTR
3. Extended CTR – CTR with and expansion procedure, arytenoid lateralization, or aytenoidectomy
* Note: some authors classify laryngotracheoplasty as a cricoid split procedures without cartilage grafting and laryngotracheal reconstruction as a procedure with grafting or segmental resection
Tracheostomy
 Tracheostomy is usually required as the inital step, particularly in the pediatric age group with acquired SGS, in the surgical management of patients with SGS. Infants with congenital SGS may forgo tracheostomy as they are often treated with primary reconstruction or are observed. Tracheostomy is often not performed in infants with congenital SGS. These patients are either treated conservatively or undergo primary reconstruction and are left intubated for approximately one weekInfants with SGS are often premature or of low birth weight. A tracheotomy allows these patients time to grow before definitive surgical treatment is performed. Cotton and Walner recommend waiting until the infant is at least 10 kilograms before performing airway reconstruction. Delayed reconstruction will also allow for optimization of pulmonary function in patients with bronchopulmonary dysplasia. It is important to note that the mortality rate of laryngotracheal stenosis is primarily due to complications from the tracheostomy of which plugging and accidental decannulation are the most common. The reported mortality rate of tracheostomy in infants and children is 2% to 5% per child per year (Lesperance, 1996).
Tracheostomy is usually required as the inital step, particularly in the pediatric age group with acquired SGS, in the surgical management of patients with SGS. Infants with congenital SGS may forgo tracheostomy as they are often treated with primary reconstruction or are observed. Tracheostomy is often not performed in infants with congenital SGS. These patients are either treated conservatively or undergo primary reconstruction and are left intubated for approximately one weekInfants with SGS are often premature or of low birth weight. A tracheotomy allows these patients time to grow before definitive surgical treatment is performed. Cotton and Walner recommend waiting until the infant is at least 10 kilograms before performing airway reconstruction. Delayed reconstruction will also allow for optimization of pulmonary function in patients with bronchopulmonary dysplasia. It is important to note that the mortality rate of laryngotracheal stenosis is primarily due to complications from the tracheostomy of which plugging and accidental decannulation are the most common. The reported mortality rate of tracheostomy in infants and children is 2% to 5% per child per year (Lesperance, 1996).
Tracheostomy may be required in adults if the patient presents in airway distress. More commonly, adults with acquired LTS have progressive symptoms over time and present early enough to allow time to plan for definitive airway reconstruction as the initial treatment with or without tracheostomy.
Once the decision to perform airway reconstruction has been decided upon, the surgeon must choose the most appropriate procedure to perform. The site, grade, and length of stenosis are the major factors in determining which surgical procedure will be used for reconstruction. The goal of all surgical procedures for LTS is to maintain vocal function and allow for early decannulation with subsequent unrestricted activity. Contraindications to reconstruction includes patients who would remain tracheotomy dependent even after adequate laryngotracheal expansion (i.e. patients with severe aspiration or BPD requiring a tracheostomy for pulmonary toilet), children with gastroesophageal incompetance resistant to surgical and medical management, and patients with an absolute contraindication to general anesthesia.
Endoscopic
Mild stenosis (Cotton-Myer grades I and II) can usually be treated with endoscopic techniques such as dilation or CO2 laser resection. Factors associated with failure of these endoscopic techniques include: previous attempts at endoscopic repair, circumferential scarring, loss of cartilaginous support, exposure of cartilage during laser excision leading to chondritis, severe bacterial infection, posterior inlet scarring with arytenoid fixation, combined laryngeal or tracheal stenosis or vertical scar length >1cm. Endoscopic dilation has had disappointing results, however, reported success rates with endoscopic laser resection of Grade I and II stenosis range from 66-80%.
Grade III or IV stenoses usually require some form of open surgical procedure. Several techniques have been described.
Anterior Cricoid Split
The anterior cricoid split (ACS) procedure was originally described for a neonate who has had multiple failed extubations instead of performing a tracheostomy (Cotton and Seid, 1980). This procedure is also used for older infants and those who are have already been tracheotomized. Indications were later expanded to patients with congenital subglottic stenosis. The lesion responsive to this procedure is a mild anterior subglottic narrowing with extensive fibrosis but a normal cricoid. ACS may also be used to decompress subglottic cysts. Strict criteria for ACS have been established by Cotton and include: extubation failure on two occasions or more due to laryngeal pathology, weight >1500g, no assisted ventilation for 10 days prior to evaluation, O2 requirements <30%, no CHF for one month prior to evaluation, no acute respiratory tract infection, no antihypertensive medications ten days prior to evaluation. The procedure is performed after direct laryngoscopic and bronchoscopic confirmation of the diagnosis. All other airway pathology must be ruled-out.
A vertical midline incision is made through the cricoid cartilage and first two tracheal rings as well as the lower thyroid cartilage. This allows the cartilages to spring open and allow edematous mucosa to drain, increasing airway size. Prolene stay sutures are placed on either side of the cricoid cartilage and the skin is re-approximated after placement of a drain. The child is then left intubated, sedated and paralyzed in the ICU for 7-14 days. Cotton has guidelines for endotracheal tube sizes for stenting and for duration of stenting based on the infants weight.
Laryngotracheal Expansion Surgery
Laryngotracheal expansion surgery involves scar division with distraction of the edges by interposition of graft material (augmentation) to widen the airway lumen. It is important to avoid removing scar which results in a large surface area of denuded mucosa and leads to restenosis. Cotton recommends augmenting the airway with grafts when the distraction of the laryngotracheal framework must be greater than approximately 3mm. There are several techniques depending on the location and severity of the stenosis. Laryngotracheoplasty can be performed with a tracheostomy and formal stenting or by using the endotracheal tube as a stent, the latter known as a single-stage LTP (SS-LTP). There are a several stents that can be used for LTS including: endotracheal tubes, Silastic sheet rolls, Montgomery T-tubes, and laryngeal stents. Laryngeal stents include: teflon stents [Aboulker stent (short or long), ETS Poirot, Paris], and silastic stents (Montgomery stents: Boston Medical Products, Boston. The primary consideration when deciding on the type of reconstruction and stent material is to provide a safe airway and adequate support for the graft. Success of LTR among, other things, is determined by the surgical procedure, including possible need for stenting; choice of type and length of stent; and duration of stenting. Choosing the appropriate method for stenting requires considering consistency of stenosis, altered anatomy, size, location and stability of grafts when used for sugical repair and host tissue healing factors (Zalzal, 1988)
Autogenous costal cartilage is the material of choice for grafting. Many other materials have been used for grafting including auricular, hyoid and thyroid cartilage and bone. Cartilage has much less resorption over time compared to bone. Although bone provides good structural support, grafts in the airway do no bear a lot of stress or weight.
Anterior laryngofissure with anterior lumen augmentation
This technique is good for anterior subglottic stenosis or anterior tracheal wall collapse. The lesion should not involve the glottis. Other procedures should be considered if there the cricoid cartilage is deformed or weak. Anterior grafts are made considerabley larger and thicker than grafts placed posteriorly. The perichondrium is oriented to the luminal side to allow for epithelialization. The perichondrium is also a good barrier against infection. A large external flange is created to prevent the graft from prolapsing into the airway.
Laryngofissure with division of posterior cricoid lamina
This is indicated for patients with posterior subglottic stenosis, posterior glottic stenosis that extends to the glottis, complete or circumferential stenosis, or if there is significant cricoid deformity. Division of the anterior and posterior cricoid must be carried out for this procedure. If possible, one should avoid a complete laryngofissure to to avoid damaging the anterior commissure, however this is often needed for posterior glottic involvement for access. The posterior cricoid cartilage is incised in a manner that is vertically oriented to the cartilage to allow maximal purchase for the graft. The incision is extended superiorly to the interarytenoid area and inferiorly 5 to10 mm into the membranous trachea. The graft is elliptical in shape. It should not be too thick as it can cause swallowing difficulties and can lead to aspiration. The width of the graft is determined by the desired distraction of the cut edges of the incised posterior cricoid cartilage. 0.05 to 1.00 mm of distraction can be obtained for each year of age, up to 1 cm. It is sutured in place with absorbable suture on a small cutting needle. The knots should be buried so that they remain extraluminal to prevent development of granulation tissue. Long-term stenting is usually necessary (3-6 months).
Laryngofissure and division of posterior cricoid lamina with anterior and posterior grafts.
This should be used for patients who have SGS similar to those above but with a significant amount of stenosis posteriorly such that grafting is necessary to maintain the adequate separation.
Once the grafts have been sutured into place in any of the above procedures, the decision must be made on whether it should be single or double-staged. Cotton and Walner (1999) recommend a double-staged procedure for patients with severe stenoses, history of reactive airway, or poor pulmonary function. This should also be considered at institutions with inadequate intensive care facilities. Double-stage procedure implies placement of stent above the tracheostomy tube instead of using an endotracheal tube as the stent (single-staged procedure). Once this decision is made, the strap muscles are closed to provide blood supply to the outer surface of the anterior graft.
Segmental resection/Cricotracheal resection (CTR) with thyrotracheal anastomosis
The first CTR was performed by Conley in 1953 in a patient undergoing surgery for chondroma of the cricoid cartilage. It was later popularized by Ogura and powers (1964) as a technique for treatment of traumatic stenosis. In the 1970s it became the treatment of choice in adults with acquired subglottic stenosis from long term intubation. Until recently, surgeons were reluctant to perform this procedure in the pediatric patients because of the risk of anastamotic dehiscence and recurrent laryngeal nerve injury, and disturbing the normal growth of the larynx. The first successful CTR performed in a child was occurred in 1978 (Savary). It wasn’t until 1993, however, that the first series of 15 pediatric patients treated with CTR for severe LTS was published. Multiple subsequent series have reported using CTR for severe LTS with good outcomes (Monnier et al., 1995, Monnier et al., 1998, Stern and Cotton, 1999).
This technique is indicated if there is severe deformity of the cricoid making grafting very likely to fail. Most say that there must be at least 10 mm of normal airway below the glottis, however Cotton states that the resection can be up to the vocal folds but to expect prolonged edema. This technique is technically difficult due to the close proximity of the vocal cords and recurrent laryngeal nerves. Stenosis less than 4 cm can be resected by laryngeal release and cervical tracheal mobilization. Stenting is not required and the trachetomy tube can ususally be removed at around 4 weeks.
Post-operative Care
Patients undergoing open laryngeal surgery for SGS require post-operative care in an ICU that has an intensivist and staff familiar with the specialized care these patients require.
Since most of the children undergoing open laryngotracheoplasty have more severe SGS they have usually had a tracheotomy for a while. Children who undergo laryngotracheoplasty with stenting typically only require a few days of care in the hospital to be sure that the parents are educated about tracheotomy care and have all necessary equipment at home. Since they have a stent in place, they should be given antibiotics and antireflux medications should also be continued. The length of time a stent should be kept in place depends on what the airway requires. If stenting is needed only to hold a graft in place while healing, one week may be all that is needed. On the other hand if the stent is used to counteract scar formation and maintain the lumen, months of stenting may be required. Stents should be monitored ever 3 to 4 weeks for displacement and granulation tissue development. Once removed, the patient should undergo repeat endoscopy every 2 weeks until healing is complete at which time decannulation is considered.
Infants and children who undergo either ACS or one-stage LTP require more intense post-operative care in the ICU. They usually stay intubated for 7-14 days with the endotracheal tube acting as a stent. The patient requires heavy sedation with or without paralysis. The patients should be treated with broad-spectrum antibiotics and antiflux medications. Chest physiotherapy and log-rolling the patient every 4 hrs. is important to prevent atelectasis, pneumonia, and pressure sores. The majority of patients do not need continuous paralysis. The patient undergoes extubation when an air leak develops. An audible air leak at a pressure of 20cm H20 is a good prognostic indicator for successful extubation. Decadron (1mg/kg) is usually given 12 hours before extubation and for 5 days after.
Most children with LTS have documented GER prior to treatment and therefore are already on an antireflux medical regimen. This should be continued in the peri and postoperative period. Patients (children and adults) without symptoms of reflux prior to treatment and those with a negative reflux workup should be treated in the peri-operative period. Some authors suggest continuing treatment up to three months after surgery in theses patients.
Complications are infrequent but can include: atelectasis, pneumonia, malpositioned endotracheal tube, accidental extubation, occluded ET tube, wound infection, granulation tissue, restenosis, and tracheocutaneous fistula. Some complications related specifically to ACS and one-stage LTP that require prolonged intubation with sedation include narcotic withdrawal and transient skeletal muscle paralysis related to the prolonged muscle relaxation.
Outcomes
The goal of management of subglottic stenosis is decannulation. Success rates are dependent on the cause of the stenosis, the number of previous failed attempts, the status of the remainder of the airway, especially the glottis and the severity of the stenosis. Cotton has reported an overall pediatric LTR success rate of 92%, 97% for Grade II, 91% for Grade III, and 72% for Grade IV.
Bailey (1988) reported results of 131 pediatric airway reconstructive procedures. He had a 92% success rate with patients who underwent laryngotracheoplasty procedures (no grafting) and 80% success with patients who underwent LTR (with grafting). He did not report on use of any grading system.
Lano (1998) reviewed 41 cases of LTR in adults. He classified patient lesions with all three previously mentioned grading systems (Cotton-Myer, McCaffrey, and Lano). Over all 80% of patients were decannulated. He found that surgical outcome significantly correlated with the Lano and McCaffrey grading system, however, not with the Cotton-Myer system. He had 94%, 78%, and 20% success rates with Lano stage I, II, and III respectively. Interestingly, the best predictor of surgical outcome was obtained by multiplying the value from the Lano and the Cotton-Myer systems. Lano suggest that the reason for this is that his classification, which grades the extent of the lesion, multiplied by the Cotton-Myer system, which grades the severity of the lesion, provides a three-dimentional rating which is more characteristic of these lesions.
Lano’s study was the first to report on the success of LTR with respect to diabetes. 75% of patients with type I or II diabetes who underwent LTR failed decannulation. The most common cause of failure in these patients was exuberant granulation tissue and restenosis with thick scar formation. One had dehiscence of the anastomotic site and requirred a T-tube to maintain an airway. Impairment of wound healing secondary to decreased microvascular blood flow and bacterial overgrowth probably account for these findings.
Monnier et al. (1999) has reviewed the experience with CTR at the Department of Otolaryngology at the University of Lausanne, Switzerland. 69 CTRs were performed (48 infants and children and 21 in adults). 95% and 100% of the pediatric and adult patients, all of whom had Cotton-Myer grade III or IV stenosis, were successfully decannulated . Stern and Cotton (1999) reported on 38 pediatric patients who underwent CTR for severe LTS (grade III and IV). 33 patients were successfully decannulated. Complications preventing decannulation in this study included one patient with persistent aspiration, three who restenosed, one with arytenoid prolapse, and one with recurrent layrngeal nerve injury. Overall, 94% successful decannulation has be reported in the literature when CTR is used in pediatric patients with severe LTS (Monnier, 1999).
Grillo (1992) has reported his experience with 80 CTRs. He reported that 97% of patients underwent successful decannulation. He further subdivided patients into groups depending on their voice quality and exercise tolerance. 22.5% had an excellent outcome defined as a normal voice and no activity restriction. 60% had a good outcome in which patients suffered only slight lessening of maximum volume of voice, slight hoarseness that did not impede vocal use, slight weakness of voice after prolonged use, diminished ability to sing, and adequate breathing for all normal activities. 10% had satisfactory results which were those with a hoarse voice and either slight wheezing or shortness of breath on exercise, not sufficient to impair usual activities.
Dysphonia following laryngotracheal reconstruction has not been extensively studied. Many authors report that a functional voice is restored in most patients. MacArthur et al. published a series of 12 patients (average age 6yrs) who underwent LTR. Most patients underwent both endoscopic evaluation and speech analysis. They found that 78% of the children had altered anatomy, 44% had altered function and 100% had decreased voice quality. They concluded that children with high grade subglottic stenosis and a history of multiple prior surgeries are at risk for a poor voice outcome after laryngotracheal reconstruction.
Prevention
Preventative measures used to reduce the likelihood of developing SGS are directed at the those events which are known or postulated to cause or exacerbate this problem. The endotracheal tube (ETT) is well established as the most common cause of SGS. As mentioned before, the size and movement of the tube, multiple and traumatic intubations, high cuff pressures, and duration of intubation all contribute to increase damage to the delicate mucosal lining of the airway. It is therefore important for the primary physician, anesthatist and/or intensivist to be aware of SGS as a complication of endotracheal intubation. This starts with education. From there, minimizing the amount of trauma to the airway is key.
Choosing the appropriate size ETT should be determined by the patients age and size. If unsure, a smaller ETT can be inserted and if the leak pressures are to low, a half size larger tube can then be placed. The ideal size tube in children allows an air leak at an inspiratory pressure of
20 cm H2O. Using this guideline, Cotencin and Narcy (1993) found a very low rate of post extubation stridor in neonates (5/247 patients) with none requiring surgical therapy for stenosis. In adults, a leak should be heard at a peak pressure of 20-25 cm H20. In addition, cuff pressure testing by a respiratory therapist should be routine in an intensive care setting. Pressure should be kept below 25 cm H2O.
Blind intubations should always be avoided if possible. If direct laryngoscopy is difficult, fiberoptic intubation should be attempted. Once a patient is intubated, multiple measures should be taken to prevent the tube from moving. These measures are usually carried out by an anesthesiologist or an intensive care unit respiratory therapist. It is particularly difficult to prevent tube movement in nonparalyzed patients with neurologic injuries or in children. Accidental extubations should be avoided at all costs as these often result in traumatic reintubations. The number reintubations can be minimized by appropriately evaluating the patient with weaning parameters and establishing the presence of an air leak.
There is no definitive safe period for intubation. 5 to 10 days of intubation is generally acceptable in adults after which time a tracheotomy should be considered. Premature infants have much more pliable cartilages allowing for longer periods of intubation. Lesperance and Zalzal (1996) recommend performing a tracheotomy in premature infants after 50 days of intubation.
Most patients who are intubated in the intensive care unit receive intravenous antireflux medications. This is important because these patients are predisposed to reflux. Nasogastric tubes act as a stent and allow gastric contents to breach the upper and lower esophageal sphincters. If possible, the head of bed should be elevated to allow gravity to work for the patient.
In the past 30 years there has be a reduction in the incidence of LTS particularly in the neonates. Seven studies looking at the years of 1971-1979 found an incidence of neonatal SGS of 0.9% to 8.3%. Nine studies looking at the years of 1980 to1989 reported an incidence of neonatal SGS of 0.0% to 4.2% and 4 studies describe patients cared for between 1990-1999 and reported an incidence of neonatal SGS of 0.0% to 0.63%. Overall the incidence today has been estimate to be 0% to 2% for intubated neonates (Walner, 2001). This compares to incidence estimates in the 1960s of upwards of 22%. This reduction has most likely resulted from better education of physicians and nurses and an increasing awareness of the factors contributing to the development of SGS. Routine use of artificial surfactant and systemic steroids in premature infants as well as the increased use of CPAP when possible has also contributed to the decline in the incidence of SGS.
SUBGLOTTIC STENOSIS IN WEGENER’S GRANULOMATOSIS
In 1931, Heinz Klinger of the University of Berlin first reported two patients who died having prolonged sepsis with inflammation of blood vessels scattered throughout the body. Five years later, Friederic Wegener in Breslau described a distinct syndrome in three patients. These patients were found to have necrotizing granulomas involving the upper and lower respiratory tract. In 1954, seven more patients were described, resulting in the establishment of the definite criteria for the diagnosis of the disease described by Wegener.
Wegener joined the Nazi Party in 1932. As a high ranking military doctor he spent some of WWII in a medical office near a Jewish ghetto in Lodz, Poland. There is speculation that he participated in experiments on concentration camp inmates. After his Nazi past was discovered in 2000, the chest physician group began a movement to rename Wegener’s granulomatosis. Dr. Friederic Wegener died in July of 1990 at the age of 83.
Wegener’s Granulomatosis (WG) is a necrotizing granulomatous vasculitis of autoimmune origin. The disease has a predilection for the upper and lower respiratory tracts and the kidneys. In the sinonasal tract, the vasculitis can cause sinusitis, nasal crusting, epistaxis, septal perforation and saddle-nose deformity. In the lungs, the disease may lead to pneumonitis and hemoptysis. In the kidneys, a crescentic glomerulonephritis develops, eventually leading to renal failure. Other manifestations include skin lesions, arthritides, conjunctivitis, and other non-specific systemic inflammatory problems. Subglottic stenosis is a potentially life-threatening manifestation of Wegener’s granulomatosis. This narrowing of the upper airway at the level of the cricoid cartilage and/or upper tracheal rings presents a management dilemma.
Subglottic stenosis (SGS) is reported to occur in 16-23% of patients with Wegener’s Granulomatosis. (Langford) It has been reported to occur more often in females. (Shokkenbroek, Gluth) The median age at diagnosis is 26. (Langford) and is more likely to occur in patients diagnosed with Wegener’s before age 20. (Langford) Patients with WG and SGS tend to have more sinus involvement and saddlenose deformity than other WG patients. On the contrary, SGS patients tend to have less lung and kidney involvement. (Langford)
The pathogenesis is unclear. Subglottic stenosis can progress in the absence of systemic disease. Few patients have WG exacerbations around the time of diagnosis with SGS. In series where patients were followed, need for repeat procedures was not related to repeated systemic disease flares. It is postulated that during flares of systemic disease, subclinical subglottic involvement occurs, which subsequently heals with circumferential scarring. (Shokkenbroek) Some authors speculate that the subglottis is vulnerable because it is a watershed area of the microcirculation. This watershed area is the junction of 2 separate embryological growth centers. (Eliachar) Exposure of respiratory epithelium to gastric contents during LPR episodes is also believed to play a role. (Gluth) Initial granulomatous inflammation is followed by circumferential scarring and airway narrowing.
Dyspnea on exertion is the most common presenting symptom. (79-82%)(Gluth, Langford) Other symptoms include voice change, stridor, or cough. Patients may have a known diagnosis of WG (50%), have other symptoms of systemic disease, or present as a new patient with airway complaints. If the diagnosis of WG is not established, an anti-cytoplasmic antibody assay should be performed. It has been suggested that ANCA titers be performed on nearly all patients with SGS. (Gluth) A positive C-ANCA assay is reported to be 91% sensitive and 99% specific for active Wegener’s granulomatosis. However, a series of patients with SGS and presumed WG showed that only 57% initially showed a positive ANCA assay. 85% of the cohort eventually became positive at a later date. (Alaani) This emphasizes the fact that SGS can be present in the absence of active disease. It also suggests that ANCA assay be repeated serially if there is diagnostic uncertainty. The presence of ANCA in serum can be detected by indirect immunofluorescence or by radioimmunoassay. Indirect immunofluorescence reveals a pattern of staining that can be more specific for a particular disease.
The “cytoplasmic” pattern (c-ANCA) is associated with ANCA reacting with a 29 kD protein from the azurophilic granules of the neutrophil.
The “perinuclear” (p-ANACA) pattern is associated with ANCA reacting with myeloperoxidase. Radioimmunoassay of ANCA titers can be used as an initial screening test and to monitor therapy and detect flare-ups of systemic disease. The airway should be fiberoptically examined in the clinic setting. Topical laryngeal anesthesia may be necessary to visualize the sublottic lesion. In the majority of cases (75%), the stenotic segment has the appearance of a mature scar and lacks acute inflammatory changes. (Gluth) CT scans will help to delineate the extent of the lesion but should not be used for primary diagnosis. The stenosis can be graded using the Cotton-Myer classification scheme for grading circumferential subglottic stenosis.
• Grade I – Obstruction of 0-50% of the lumen obstruction
• Grade II – Obstruction of 51-70% of the lumen
• Grade III – Obstruction of 71-99% of the lumen
• Grade IV – Obstruction of 100% of the lumen (ie, no detectable lumen)
Histological proof of WG is obtained by finding vasculitis, necrotizing granulomata and giant cells in biopsy material. But, in general, biopsies of the subglottic lesion are not sensitive for the detection of WG. Only 5-15% of subglottic biopsies performed on patients with positive ANCA titers and SGS return changes consistent with WG. (Gluth, Langford) In contrast, nasal biopsies on a similar cohort of patients yielded 82% sensitivity for WG. (Gluth) Pulmonary function tests are abnormal in 60% of patients with subglottic stenosis. (Langford) Flattening occurs in the flow-volume loop in both the inspiratory and expiratory portions, giving it a box-like appearance. However, PFTs may not detect less severe stenoses and should never be used for primary diagnosis. An abnormal flow-volume loop is correlated with need for surgical intervention. (Langford)
Treatment of subglottic stenosis should be considered based on the presence of symptoms combined with objective evidence of tracheal narrowing. Other parameters, such as active systemic disease or a change in ANCA titers should never be used as consideration for procedures. Treatment options include immunosuppression, tracheostomy, endoscopic dilation, intralesional steroids, laser procedures, cold knife lysis, open surgical procedures, and airway stents.
Drugs such as corticosteroids, cyclophosphamide, and azothioprine has revolutionized the treatment of WG, with a dramatic reduction in mortality. However, subglottic lesions are generally unresponsive to systemic agents. 49% of SGS cases are diagnosed while a patient is on active therapy. (Langford) Success rates of medical therapy in relieving the obstruction vary from 22%-26%. (Gluth, Langford)
Life-threatening airway obstruction may require tracheostomy as a temporizing or permanent measure. Tracheostomy is necessary in 8 – 60% of cases. (Langford, Schokkenbroek, Gluth, Alaani) Often, success of other therapies is measured by decannulation of the tracheostoma.
Endoscopic procedures have been variably successful at managing this airway lesion. Dilation tracheoscopy can be performed using a Groningen optical dilatation tracheoscope (Karl Storz 1033R)(Fig 1) fitted with a 30 cm Hopkins telescope. Under general mask anesthesia, the scope is introduced. The beveled, fenestrated tip is designed to ensure ventilation as the scope is advanced through the stenosis. The conical design allow the scope to be advanced up to its wider portion. The scope is left in place for 5 – 10 minutes. Dilation tracheoscopy has been shown to be effective the majority of the time for Cotton-Meyer grade I-II in the short term. Repeat procedures are commonly necessary. No complications have been reported with this procedure. The small number of reported cases (9) should be considered. (Shoekkenbroek)
Traditional dilation techniques, combined with intralesional steroid injection have been reported. Under suspension laryngoscopy and spontaneous ventilation, graduated dilators are used to dilate the trachea. Next, methylprednisolone injections are performed in a a 4-quadrant, submucosal pattern. Perioperative, systemic steroids are also used. 20 patients received this treatment and were followed for a median of 35 months. The median number of treatments required was 3. Six patients required only 1 procedure. One patient required 22 procedures.. All patients who began therapy with a tracheostomy were eventually decannulated. No patients required a new tracheostomy.
In another series, 21 patients with WG and significant SGS were studied. These patients were treated with intralesional steroid injection combined with mechanical dilation and cold-knife lysis of the lesion. Under suspension laryngoscopy and jet ventilation, methylprenisolone was injected submucosally in 4 quadrants. Lysis of the stenotic ring was then performed by making radial incisions with a laryngeal microsickle knife. The stenosis was then serially dilated with Maloney bougies or a Foley catheter. Topical mitomycin C was variably used. (Hoffman)
Patients with prior procedures (mostly laser) averaged more procedures to obtain adequate airway patency and with a shorter interval between procedures. The authors comment that, subjectively, lesions seen in patients who had undergone prior laser procedures were severe, extensive, and thickly fibrotic. No new tracheotomies were necessary in either group. (Hoffman) The most difficult lesions were found in the 6 patients with prior tracheotomies, presenting with multilevel stenoses, cicatrix formation, vocal cord fixation and arytenoid damage. In these patients, widening of the subglottic region alone was not sufficient. 4 of the 6 patients achieved decannulation by means of various laryngotracheoplastic techniques which were not specified. The authors comment that laser procedures cause extensive scarring and thus patients are more difficult to manage afterward. However, these patients may represent a cohort with more severe disease, who would be more difficult to manage anyway. There is no way to differentiate hether the scarring is from prior laser procedure, or from more extensive inflammation in the airway .
Although abhorred by other authors, laser treatment of SGS has been performed and reported on. For lesions close to the vocal cords, in 2 patients, CO2 laser was used with microsuspension laryngoscopy to resect the stenosis. For lesions far from the cords (>1 cm), in 3 patients, Nd:YAG laser was used with through a fiberoptic bronchoscope, under local anesthesia. (Shvero) 4 out of these 5 patients are reported to have favorable outcomes or decannulation during their follow up period of 6-60 months. However, all patients required multiple treatments (2-18) In addition, one patient required placement of a subglottic stent. (Shvero)
Open surgical procedures have been performed with success. Most commonly performed is the laryngotracheal reconstruction with costal cartilage graft (LTR). It is recommended to defer LTR until a patient is in a quiescent phase of the systemic disease. This should be monitored by serum ANCA titers, CRP, and ESR. It is also recommended that surgery be deferred until a patient is not actively taking steroids or other immunosuppressant medications. (Gluth) A small series of Wegener’s patients who received laryngotracheal reconstruction for SGS shows excellent results. 6 of 6 patients were decannulated from their tracheostomy after the LTR. There were no cases of graft failure even in 3 of the patients in whom reactivation of systemic WG occurred. 1 complication of graft displacement occurred. (Gluth)
The use of airway stents to maintain airway patency in SGS and WG is controversial. Some authors have advocated deployment of a Nitinol (nickel-titanium alloy) expandable stent into the subglottic airway when all other therapies fail. Similar stents have been used with success in the distal tracheobronchial tree. A case report exists in the literature. A patient was followed for 48 months after deployment of a nitinol stent. She had a patent airway, no complications, and no ingrowth of granulation tissue. (Watters) Other authors argue that the long-term safety and efficacy of these stents has not been well established. They argue that these devices are associated with stent fracture, migration, increased granulation tissue, and even death. Stents in the subglottis also undergo movement with swallowing and saliva exposure. These factors preclude early mucosalization of the stent, as occurs in more distal portions of the airway. The foreign body reaction, it is argued, causes more granulation tissue ingrowth. (Mair- anecdotal) The argument against stents is further supported by the fact that an emergency trachotomy in the setting of a subglottic metal stent is very difficult to perform.
Based on the published research, the following treatment plan should be followed. If the airway is tenuous, perform emergent tracheotomy. If patient is stable, perform PFTs, CT scan, and fiberoptic endoscopy. 1st line therapy should be endoscopic dilation. With subsequent dilations, cold-knife lysis, and intralesional steroids should be added. Patient should be followed every 3 months with repeat fiberoptic exams and PFTs. Serial ANCA titers are not necessary from an ENT standpoint, since they correlate poorly with SGS progression. Endoscopic procedures should be repeated as necessary. If endoscopic procedures must be performed at an increasingly more frequent interval, consideration should be given to open laryngotracheal reconstruction. Subglottic stents should be avoided if at all possible.
Discussant’s Remarks: Francis B. Quinn, Jr., MD, FACS
Idiopathic Midline Destructive Disease
Today’s Grand Rounds is a fine discussion of the incidence and treatment of subglottic stenosis occurring in patients who suffer from idiopathic midline destructive disease. One may infer or doubt a causative relationship based on data regarding the absence of granulomatous histology in the stenosis itself, or by the association of positive C-ANCA titers in cases of stenosis occurring in the absence of clinical midline destructive disease.
The taxonomy of idiopathic midline destructive disease has not benefited from the attempts to identify and classify its variants, based on etiology (autoimmune, infectious, neoplastic), histology, clinical manifestations, responses to therapy (microbial, corticosteroid, chemotherapeutic, ionizing radiation) and natural history (remittent, inexorable progression, lethal).
A brief walk through the medical literature yields a collection of terms including polymorphic reticulosis, lethal midline granuloma, malignant midline reticulosis, natural killer-cell lymphoma, peripheral T-cell lymphoma, Wegner’s granulomatosis (limited type – without renal disease, and classical type – with renal disease), angiocentric T-cell lymphoma, and idiopathic midline destructive disease.
While the eponymic “Wegener’s granulomatosis” serves the vulgate, the inclusive “idiopathic midline destructive disease” will point to the disorder as a challenge to the therapist and not merely a diagnostic dead end with ineluctably hopeless prognosis.
The term “idiopathic midline destructive disease” can be considered as a collection of vectors in which every manifestation of the disease (clinical, histologic, course, etc.) is represented by data types appropriate to that manifestation (continuous, discrete, Boolean, or null). The individual patient can be mapped onto the N-space such that cluster analysis leads to a rational nosology permitting an enlightened pathophysiologic understanding of this (group of) disease(s).
SUPRAGLOTTIC LARYNGECTOMY
HISTORY
Supraglottic laryngectomy (SGL) is a surgical technique designed with the goal of complete elimination of cancer arising from the epiglottis, aryepiglottic folds, and false vocal cords while minimizing morbidity and maintaining the three primary functions of the larynx—airway protection, respiration, and phonation. Prior to its introduction by Alonzo in 1947, the primary treatment for supraglottic tumors was total laryngectomy and radical neck dissection leading to the disabilities referred to by Bocca above. Despite the functional benefits of a partial laryngectomy, general acceptance of this procedure was not immediately forthcoming. Not only did the medical community at the time question the oncologic safety of the procedure, but also Alonzo’s original description of the procedure necessitated a second stage operation to close a temporary pharyngostoma. Modifications made by Ogura in 1958 converted supraglottic laryngectomy to a one-stage procedure. Som, in 1959, reported the technique of reconstructing the surgical defect with primary reapproximation of the outer thyroid perichondrium to the tongue base—the procedure we continue to use today.
EMBRYOLOGY/ANATOMY
The larynx is comprised of three anatomic subunits—the supraglottis, glottis and subglottis. Structures that comprise the supraglottis include the lingual and laryngeal surfaces of the epiglottis, the aryepiglottic folds, the arytenoid cartilages, the false vocal folds, and the ventricle. During embryologic development these structures are derived from the buccopharyngeal anlage of branchial arches three and four. In contrast, the glottic and subglottic structures develop from the tracheobronchial anlage of the fifth and sixth branchial arches. This embryonic fusion plane, represented by a horizontal line drawn through the ventricle, is an essential anatomic concept to understanding the validity of the supraglottic laryngectomy.
Pressman’s study in 1956 lent further support to the oncologic safety of the procedure by demonstrating that dyes injected submucosally in the supraglottis did not spread inferior to the ventricle. Fibroelastic membranes within the laryngeal framework serve as functional barriers and provide the anatomic explanation for these findings. These membranes which include the thyrohyoid membrane, the hyoepiglottic ligament, the thyroepiglottic ligament, the conus elasticus and the quadrangular membrane also serve to divide the larynx into two three-dimensional compartments—the preepiglottic space and the paraglottic space. The preepiglottic space is defined superiorly by the hyoepiglottic ligament, anteriorly by the thyrohyoid membrane, inferiorly by the thyroepiglottic ligament and posteriorly by the epiglottis. The paraglottic space is bounded superiorly by the quadrangular membrane and medial piriform sinus wall, inferiorly by the conus elasticus and laterally by the thyroid cartilage. Involvement of these spaces by a supraglottic cancer—most commonly through the infrahyoid epiglottis and ventricle—has a direct effect on potential surgical management of these lesions.
The lymphatic drainage of the supraglottis was also defined by Pressman. The “lateralized” structures of the aryepiglottic folds and false cords were found to demonstrate ipsilateral lymphatic drainage while the midline epiglottis drainage pattern was bilateral. Additionally, the supraglottis has a superficial lymphatic system, which tends to flow bilaterally with less regard for site while deeper lymphatics maintain laterality based upon the site of the lesion. The lymphatic vessels travel alongside the superior thyroid artery and vein, through the thyrohyoid membrane, and empty into the jugulodigastric (level II) and midjugular (level III) lymph nodes.
SUPRAGLOTTIC CANCER
Of the nearly 13,000 new cases of laryngeal cancer diagnosed annually in the United States, 30-40% of these tumors are thought to arise in the supraglottis. The vast majority (95%) of these tumors are squamous cell carcinoma. However, tumors of salivary gland origin (acinic cell, mucoepidermoid, adenocystic) mesenchymal tumors (chondrosarcoma, fibrosarcoma, liposarcoma) and benign conditions (papilloma, necrotizing sialometaplasia, granular cell myoblastoma) can also involve the supraglottis. Like other head and neck cancers, there is a higher incidence in men and significant tobacco and alcohol use are major risk factors for the development of supraglottic squamous cell cancer. The most common site of origin is the infrahyoid epiglottis followed by the false cord, suprahyoid epiglottis, aryepiglottic fold and ventricle. Local tumor spread occurs via mucosal extension, submucosal extension, and deep invasion. Invasion through foramen that house mucus glands within the cartilage of the epiglottis allows spread to the preepiglottic space. The paraglottic space becomes involved most often by spread of disease involving the mucosa of the ventricle. Compared to glottic squamous cell cancer, supraglottic cancer has been associated with early metastasis to cervical lymph nodes. Over 50% of patients will have neck disease at presentation and over 25% of patients will have occult metastasis. Distant metastasis most commonly involves the lung and occurs infrequently (5%).
The staging system for supraglottic cancer as set forth in the 1997 American Joint Committee on Cancer is as follows:
Tumor
T1: tumor limited to one supraglottic subsite with normal true vocal cord mobility
T2: tumor involves more than one supraglottic subsite or region outside the supraglottis (vallecula, base of tongue, medial wall of pyriform sinus) or invades the glottis but with normal cord mobility
T3: tumor limited to the larynx with cord fixation or involves the postcricoid mucosa or preepiglottic space
T4: tumor extends beyond the larynx with involvement of lateral pyriform, soft tissues of the neck, oropharynx, esophagus or destruction of the thyroid cartilage.
Nodes
N1: single ipsilateral node < 3cm
N2a: single ipsilateral node > 3cm, < 6cm
N2b: multiple ipsilateral nodes, all < 6cm
N2c: contralateral nodes, all < 6cm
N3: any nodes > 6cm
Metastasis
M0: no distant metastasis
M1: any distant metastasis
Staging
I = T1, N0, M0
II = T2, N0, M0
III = T1-3, N1, M0
IV = any T4, T1-3 + N>1, any M>0
This staging system serves to group diseases with similar prognosis for use in patient counseling and treatment planning as well as providing a standardized language by which medical professionals can communicate. The majority of supraglottic tumors present with advanced disease—stages III and IV.
DIAGNOSIS/EVALUATION
Many patients with supraglottic tumors will remain asymptomatic until the tumor has reached an impressive size. Alternatively, the presenting complaint will be an enlarging asymptomatic neck mass. Signs and symptoms to address during history taking include things such as hoarseness or change in quality of voice, dysphagia, odynophagia, chronic cough and sore throat, globus, hemoptysis, otalgia, and stridor. Past medical and surgical history will provide insight about the patient’s general health. Social history not only helps to identify the potential risk factors of tobacco and alcohol use but also can provide information regarding the patient’s functional status by asking about occupation and recreational activities. Physical examination, although focused in the head and neck region, should cover all organ systems. Careful evaluation of the entire upper aerodigestive tract is mandatory to exclude the presence of any synchronous pre-malignant or malignant lesions. Palpation along the floor of mouth and posterior tongue provides important information regarding extent of disease. Visualization of the larynx may be performed with the laryngeal mirror or the flexible fiberoptic endoscope. The endoscopic exam provides the advantage of allowing examination of the larynx in its naturally suspended position. The adequacy of the airway, location and extent of the mass, and mobility of the vocal cords should be determined on exam. Palpation of the neck to determine the presence of lymphadenopathy should always be undertaken although some studies have reported the accuracy of this exam to vary between 50-90%.
Preoperative studies that should be obtained in the patient with a supraglottic mass include a complete blood count, serum chemistry panel, liver function tests, and a chest x-ray. A CT scan of the neck is felt to be the best imaging study to evaluate a supraglottic lesion. These images are most useful in defining the deep extent of tumor and allow identification of preepiglottic or paraglottic space involvement or thyroid cartilage invasion. CT scan may also reveal enlarged lymph nodes that were not detected on physical exam. It is best to perform CT scan prior to any biopsy so as not to obscure the margins of the mass with resultant edema or hemorrhage.
Prior to any definitive treatment these patients should undergo panendoscopy and biopsy under general anesthesia. Repeat palpation of the oral cavity and neck may provide additional information once the patient is relaxed. Direct laryngoscopy is performed to verify extent of the tumor and delineate involved structures in order to allow planning of appropriate definitive therapy. Biopsies must be large enough to provide adequate tissue for pathologic study. Biopsies can be taken not only of grossly abnormal tissue to provide a histologic diagnosis but also along marginal areas to provide additional information about the extent of the lesion. Esophagoscopy and bronchoscopy are performed to exclude the presence of synchronous malignancies. Once all of this information is collected, presentation of the patient at a multidisciplinary tumor board allows for discussion among professionals from a variety of specialties to determine the best treatment options for that patient.
SELECTION CRITERIA
One of the most important factors influencing the success of supraglottic laryngectomy is appropriate patient selection. Patient factors and tumor factors play an equally important role in this selection process.
Every patient undergoing supraglottic will experience at least mild temporary aspiration postoperatively. Therefore, prior to undertaking this treatment route, the patient’s cardiopulmonary reserve must be considered. Some clinicians use pulmonary function testing to determine this factor. Many feel that using the arbitrary value of FEV1 > 50% of expected is an imprecise measurement of function and instead rely more upon patient history of daily activities. Stair climbing is a simple clinical maneuver that can provide the necessary information. What one is attempting to delineate is that the patient will be able to participate in their postoperative care with early ambulation and strong cough for pulmonary toilet.
Tumor factors are equally important in the selection process and established contraindications are based upon the anatomic considerations that were discussed earlier. Tumor involvement of the thyroid cartilage or the anterior commissure, which increases the likelihood of thyroid cartilage invasion, is a contraindication for supraglottic laryngectomy. Once the inner thyroid perichondrium has been invaded, tumor spread is unpredictable and safe cartilage cuts cannot be made.
Vocal cord fixation is suspicious for paraglottic space involvement with tumor. Since this space extends inferiorly beyond the base of the ventricle it would not be completely removed with a SGL. The standard procedure can be extended to include one arytenoid but bilateral excision will result in failed rehabilitation. Similarly, resection of tumor involving the pyriform apex or postcricoid mucosa will lead to increased postoperative aspiration and dysphagia. Base of tongue involvement extending superiorly beyond the circumvallate papillae will make primary closure difficult and results in increased dysphagia and aspiration.
Despite consciencious and thorough preoperative evaluation with examination and imaging, some of these tumor factors will not be obvious until the time of surgery. Therefore, it is essential to include in the preoperative discussion with the patient the potential necessity of conversion to a total laryngectomy intraoperatively.
TECHNIQUE
A standard tracheostomy is performed prior to the definitive procedure with strict attention paid to keep this incision separated from the neck wound. A superiorly based apron skin incision is designed and flaps are elevated to above the hyoid superiorly. The inferior and posterior extent of elevation is dictated by the type of neck dissection to be performed. Most often, this will minimally involve selective dissection of levels II-IV and this will be performed prior to the SGL. The suprahyoid muscles are released from the hyoid and the infrahyoid muscles divided 1 cm below the hyoid and reflected inferiorly. The greater cornu is skeletonized bilaterally with careful identification and preservation of the hypoglossal nerves. The outer thyroid perichondrium is incised along its superior aspect and elevated inferiorly but left attached to the inferior cartilage. Next the thyroid cartilage cuts must be planned. In the past these cuts have been placed at such locations as one centimeter below the thyroid notch, the midway point between the thyroid notch and inferior cartilage border, and the junction of the upper one third and lower two thirds of the cartilage. A human cadaver study by Meiteles et al. in 1992 confirmed the safest location of cartilage cuts to be the junction of the superior one third and inferior two thirds in both men and women. Precise placement of the cuts is essential to avoid trauma to the anterior commissure and true cords. The cuts extend through the cartilage but the inner perichondrium should be left intact at this time. The cuts are extended posteriorly to include the superior cornu of the thyroid cartilage. In smaller unilateral lesions this can be modified to preserve the superior cornu on the contralateral side. This may improve postoperative rehabilitation by preserving the superior laryngeal nerve. Entry into the pharynx is dictated by tumor location but may occur through the vallecula or the contralateral pyriform sinus. Mucosal cuts begin anterior to the arytenoid on the lesser-involved side. Using heavy scissors a cut is made perpendicularly across the aryepiglottic fold inferiorly to the level of the ventricle. The scissors are then turned horizontal to the larynx with one blade in the ventricle and one through the cartilage cut. This proceeds anteriorly to the midline thereby opening the larynx somewhat like a book and allowing resection of the more involved side with maximal visualization. During the mucosal cuts it is important to attempt to leave the mucosa over the arytenoids intact. If this was not possible, all exposed cartilage should be covered using mucosa from the adjacent pyriform sinus. The wound is then closed by reapproximating the thyroid perichondrium to the tongue base. If the perichondrium is insufficient, drill holes are made through the thyroid cartilage for suture placement. It is important that the bites through the tongue base include the deep musculoaponeurotic layer to increase strength of the closure. The stitches are delayed until the shoulder roll is removed and the head flexed and then tied in sequence. This effectively reduces tension on the closure, which is then reinforced by approximating the suprahyoid and infrahyoid musculature. Suction drains are inserted and the skin closed in layers.
Modifications of the procedure advocated by some authors include suspending the remaining thyroid cartilage to the mandible and inferiorly releasing the larynx by dividing the infrahyoid strap muscles. These additions have not demonstrated significantly improved postoperative rehabilitation. The simultaneous performance of a cricopharyngeal myotomy is controversial. Some clinicians believe that it improves postoperative swallow while others state that it increases the risk of postoperative aspiration by increasing the propensity for laryngopharyngeal reflux.
The standard SGL can be extended to include one arytenoid, part of the pyriform, or the tongue base. If an arytenoid is resected the free edge of the true cord is reapproximated to the body of the cricoid posteriorly in the midline. Tumor involvement of the vallecula necessitates extended tongue base resection. In this case the pharynx is entered through the pyriform and resection occurs from inferior to superior.
The circumvallate papilla is the superior most extent of safe resection. Small lesions that involve the superior aspect of the anterior or medial pyriform may be resected in continuity with a SGL. These extended procedures are all associated with increased aspiration and dysphagia. Patients selected for these operations must have excellent cardiopulmonary reserve and be extremely motivated.
POSTOPERATIVE CARE/REHABILITATION
Functional outcome after conservation laryngeal surgery is dependent upon specialized postoperative care. Primary issues encountered in post-SGL patients are tracheostomy decannulation and return to oral intake. The cuff on the tracheostomy tube is initially left inflated to help minimize early aspiration associated with expected postoperative edema. As soon as the patient demonstrates a strong cough and ability to protect the airway, the cuff is deflated. If this is tolerated then the trach is downsized. Once trach plugging can be tolerated for 24 hours then the patient can be safely decannulated. It is desirable to decannulate the patient prior to beginning oral feeding so as to prevent aspiration secondary to laryngeal tethering at the trach site. A study conducted in Spain in 1996 revealed an inability to decannulate to be a significant post op complication. Nearly 24% of patients with supraglottic lesions and 50% of patients with base of tongue lesions required permanent tracheostomy. Factors associated with this complication were advanced stage (T3, T4) and advanced patient age (>65 y/o). Similar rates between 20-30% have been demonstrated in other studies. In contrast, a study of the MD Anderson experience reported an early decannulation rate of 80% and 100% eventual decannulation. This study did however employ strict selection criteria that excluded many patients with advanced disease. Prolonged time to decannulation was associated with extended procedures.
Voice rehabilitation after SGL generally is successful. A study from the University of Florida reported return of voice in all of their 40 patients who underwent SGL. Further evaluation of voice quality was not reported. Other reports looking at voice quality report 93% speech intelligibility following SGL.
Probably the most difficult aspect of recovery from SGL is resumption of safe oral intake. Patients are initially maintained on tube feedings while they begin to learn to swallow their own saliva. Speech therapy consultation is warranted at this juncture to begin patient teaching of the supraglottic swallow. This is not an easy maneuver to master and requires significant time from the therapist and significant motivation from the patient. The supraglottic swallow consists of six steps. The patient first takes in a deep breath and then performs a valsalva maneuver, which aids in glottic closure. Food is placed in the mouth and the patient swallows then coughs to clear the laryngeal inlet. This is followed by a second swallow and cough prior to the next inspiration. It is best to start with soft or pureed foods rather than clear liquids. Once the patient is able to take thin liquids, carbonated beverages seem to be better tolerated. As many as 10% of patients undergoing standard SGL will have significant postoperative swallowing problems requiring permanent feeding tubes. Extended procedures are associated with both a prolonged time to swallow recovery and a decrease in patients that achieve normal to swallow to only about 57%. Factors that appear to be associated with poor postoperative swallow and aspiration based on videofluoroscopic evaluation are low laryngeal position and delayed oropharyngeal transit. This stresses the importance of superior and anterior repositioning of the larynx after tumor excision.
Perhaps the most devastating complication after SGL in the necessity to perform a total laryngectomy secondary to chronic aspiration. The conversion rate in the literature varies from 2.5 to 12.5% of cases. Factors associated with increased likelihood of total laryngectomy were again advanced stage of disease requiring extended procedures and patient age over 65 years.
ENDOSCOPIC LASER SURGERY FOR SUPRAGLOTTIC CANCER
The concept of endoscopic management for supraglottic lesions began in 1939 when Jackson described the use of a tubed laryngoscope and punch biopsy forceps to resect tumors of the suprahyoid epiglottis. Technologic advances such as the development of the operating microscope in the 1950’s, the introduction of suspension microlaryngoscopy in the 1960’s, and the use of the carbon dioxide laser for endolaryngeal surgery in the 1970’s led to the further expansion of this concept.
Potential advantages of endoscopic vs. open surgery include no need for tracheostomy, shorter operating time, decreased incidence of pharyngocutaneous fistula, no neck incisions, and earlier rehabilitation of swallow. Disadvantages include the need for specialized equipment, prolonged healing time as the defect is allowed to heal secondarily, and the potential need for a second procedure if a staged neck dissection is to be performed.
The use of endoscopic laser resection with an intent to cure is dependent upon size, location and extent of the tumor. T1 and T2 lesions located on the suprahyoid epiglottis, aryepiglottic fold and vestibular fold with minimal preepiglottic and no paraglottic involvement may be approached with endoscopic resection. Tumors that arise on the infrahyoid epiglottis and false cord are less amendable to endoscopic resection secondary to their tangential relationship to the distal lumen of the laryngoscope. The previously mentioned subsites, on the other hand, are perpendicularly oriented to the laryngoscope which facilitates tumor cuts by the laser.
The most important factor in endoscopic laser surgery is adequate exposure. The tubed laryngoscopes used for glottic surgery expose only a small surgical field. Steiner’s bivalved laryngopharyngoscope and Zeitels’ adjustable supraglottiscope more than triple the operative field and allow optimal exposure. The superior blade is placed in the vallecula and the lower blade pushes the endotracheal tube against the posterior pharyngeal wall. The laryngoscope is repositioned as needed to maintain optimal exposure throughout the case.
The carbon dioxide laser is the laser of choice for endolaryngeal surgery. Advantages of the CO2 laser include its superficial effect which helps to minimize damage to surrounding normal tissue. Coupling of the laser to a microspot micromanipulator allows for precise tissue cutting. The laser is hemostatic for vessels .5-1.0mm in diameter. And, the no touch tissue destruction allows the surgeon to observe the lasers effect on the tissue layer by layer.
Small supraglottic tumors on the suprahyoid epiglottis or aryepiglottic fold may be resected en bloc with the endoscopic laser. The majority of supraglottic cancers, however, must be excised piecemeal. The epiglottis is split sagitally in the midline with resection of the suprahyoid division first followed by the infrahyoid component. The preepiglottic fat is then encountered and removed until the thyroid cartilage is identified. Resection is then continued inferiorly to include the aryepiglottic folds and false cords as needed. Frozen sections are taken from the specimens and re-resection performed for positive margins. The defect is allowed to granulate and heal by secondary intention. Potential complications of endoscopic excision include intraoperative or postoperative hemorrhage and laryngeal chondritis in exposed cartilage.
A recent study out of Germany by Ambrosch et al reported a 5-year local control rate of 100% for T1 and 89% for T2 supraglottic cancers. Similar control rates are cited in other reports on endoscopic resection experience. This control rate is similar to that for open horizontal SGL and slightly better than that for primary XRT therapy for similar lesions.
Functional recovery in terms of post operative swallow is more rapid following endoscopic excision. Ambrosch reports a mean of 6 days of post operative nasogastric intubation which is similar to Eckel’s experience in which the majority of patients required an NGT for only 10 days post op. This is a significantly shorter time period for tube feeding than that reported for open SGL. Endoscopic tumor resection maintains the normal suspension of the larynx by preservation of the hyoid and strap muscles. This, in addition to sensory preservation through the superior laryngeal nerves, facilitates early recovery of swallow. Eckel reports that with endoscopic surgery 40 of 46 patients did not require tracheostomy at all any time during their treatment. Four of the remaining 6 were decannulated within 9 weeks of surgery. Both authors report normal voice after surgery.
SURGERY VS. RADIATION THERAPY
Treatment options for patients with supraglottic cancer include primary surgery, primary radiation therapy (XRT), or adjunctive pre- or post-operative radiotherapy. The decision for surgery, radiation, or both must be made on an individualized basis.
Advantages of surgery over XRT include less long term tissue damage, improved follow up examination, reservation of radiation therapy for recurrent cancer, and definitive pathologic evaluation of the extent of disease. Disadvantages of surgery include the postoperative rehabilitative issues mentioned previously as well as the potential necessity of intraoperative conversion to total laryngectomy. Advantages of radiation therapy include avoidance of operative morbidity/mortality and reservation of surgery for salvage of treatment failures. Disadvantages include long term tissue damage with associated difficulty in follow up exam, the potential for chondroradionecrosis, XRT cannot be used again for recurrent or secondary head and neck cancer, and post-XRT surgical salvage is more difficult and likely to necessitate total laryngectomy rather than a conservation procedure.
Most clinicians agree that both primary XRT and surgery offer good control of early disease. Conservation surgery is associated with a lower recurrence rate (5%) compared to primary XRT (23%) as reported by Spriano et al. But, they also noted a trend toward improved local control with new hyperfractionation protocols. They also recognized that although surgery may offer slightly improved control, many patients may not qualify for SGL and therefore primary XRT offers a reasonable organ preserving option.
The control rates for primary XRT used for stage III and IV disease are significantly lower than with surgery—T3 = 40% vs. 85%, T4 = 30% vs. 80%. Unfortunately, advanced disease may also make the patient unsuitable for conservation surgery. In these cases, in order to achieve improved disease control, more extensive surgery may be required and the patient will have to sacrifice function.
Alternatively, those patients with advanced disease but good function may benefit from combined surgery and XRT although this regimen is not without morbidity. Primary tumor factors that warrant postoperative XRT include close or positive margins (neck disease factors will be discussed later). Steiniger et al reviewed 29 patients undergoing supraglottic laryngectomy. Seventeen patients had postoperative XRT while 12 had surgery only. Evaluation of overall survival demonstrated no difference between the two groups but increased morbidity was noted in the XRT group (delayed decannulation or oral feeding, aspiration, airway obstruction). A confounding factor in this study is that a significantly higher percentage of patients with advanced stage disease were in the combined therapy group (76% vs. 25%). In contrast, the MD Anderson study and the multicenter study reported by Rademaker did not note XRT to cause additional morbidity in terms of decannulation or postoperative swallow.
One additional therapeutic option which is continuing to evolve is the use of concomitant chemotherapy and radiation therapy. Patients with T3 and T4 supraglottic squamous cell cancer may elect to undergo this organ sparing protocol which involves daily XRT to a dose of 5,000cGy and two cycles of cisplatin and 5-fluorouracil. The patient is then examined to assess tumor response. Those patients who have had only partial response are offered salvage surgery while those with complete response receive 2,000cGy more of XRT. With this protocol up to 60% of patients retain functional voice and swallow. Potential complications include the need for permanent trach or g-tube as well as the inherent morbidities of chemotherapy and XRT. Preliminary information indicates comparable survival rates between these patients and those treated with total laryngectomy and postoperative XRT.
MANAGEMENT OF THE NECK
The most important factor influencing long-term survival in supraglottic cancer patients appears to be involvement of the cervical lymph nodes. Either occult or clinically obvious neck disease is associated with a 50% decrease in survival. Opinions as to how to best address the N0 neck is highly variable. The first question is what modality to use to address the neck, surgery or XRT. Most agree that single modality therapy is desirable if at all possible. Simultaneous XRT delivered to a clinically N0 neck along with the primary site offers good control rates. If, however, the patient is to have surgery for the primary then the N0 neck is best treated surgically. This poses the second question, which is which side of the neck to dissect. Hicks et al studied patterns of neck disease in patients with supraglottic tumors and found that 30% of patients with clinically N0 necks had occult disease. The incidence of bilateral disease was found to be 44%. Lutz et al looked at patterns of recurrence with supraglottic patients. Their study included both N0 and N+ subjects but found that recurrence in the unoperated neck was the most common site of local-regional failure irregardless of the primary tumor site being midline or lateral. Studies such as these have led many to believe that bilateral neck dissection should be performed for the clinically N0 neck. The final question would then be, what levels of the neck to include in the dissection. The dye studies performed by Pressman indicated the lymph nodes in level II and III to be most likely involved with metastasis from the supraglottis. From this information it would seem reasonable to perform selective dissections of levels II-IV. However, Hicks et al found that of the N0 necks with occult metastasis, 82% had level I disease. This data necessitates a level I-IV selective neck dissection. Treatment of the N+ neck is less controversial and involves either selective I-IV dissection for limited neck disease or radical/modified radical neck dissection for more extensive nodal involvement. Postoperative XRT to the neck is used if pathology demonstrates multiple positive nodes or extracapsular spread.
CONCLUSION
“I conclude by saying that many things have changed in the surgical management of supraglottic cancer, but changes concern the techniques and not the principles of cancer surgery, that is, the necessity of being radical in both the primary and the neck. Supraglottic laryngectomy combined with functional elective or curative neck dissection is fully in line with those principles and it represents a priceless contribution to saving more lives while sparing mutilation…I am persuaded that the solution to the problem of supraglottic cancer in its entirety is still in the surgeon’s hands, provided that we remember that we are waging a war against cancer in the larynx and in the lymph nodes of the neck, and not against the larynx and the neck.”
—Ettore Bocca