Otology 3
Treatments for Meniere’s Disease
Meniere’s Disease was first described by Prosper Meniere in 1861 as a disease complex associated with vertigo, deafness, nausea, vomiting and aural fullness. Meniere postulated a labyrinthine origin of these symptoms. In 1871, Knappin theorized that a dilatation of the membranous labyrinth was responsible for these symptoms. In 1938, Hallpike and Portman confirm endolymphatic engorgement, or hydrops causing dilatation of the membranous labyrinth when they histologically examined temporal bones. Since that time, despite a great deal of research into the topic, we have yet to have a much deep understanding of the disorder than did Meniere. A great deal of controversy exists surrounding the pathophysiology of Meniere’s disease and treatments for the disorder.
The American Academy of Otolaryngology and Head and Neck Surgery has refined the definition of Meniere’s several times. In examining the literature on Meniere’s disease, it is important to have an understanding of these definitions. It is useful for researchers to use the definitions to standardize reporting of results. The most recent revision was set forth by the AAO-HNS Committee on Hearing and Equilibrium in 1995. The definitions are listed below:
- Possible Meniere’s disease
- Episodic vertigo of the Meniere’s type (>20 minutes, associated with horizontal rotatory nystagmus) without documented hearing loss, or
- Sensorineural hearing loss, fluctuating or fixed, with dysequilibrium but without definitive episodes
- Other causes excluded
- Probable Meniere’s disease
- One definitive episode of vertigo
- Audiometrically documented hearing loss on at least one occasion
- Tinnitus or aural fullness in the treated ear
- Other causes excluded
- Definite Meniere’s disease
- Two or more definitive spontaneous episodes of vertigo 20 minutes or longer
- Audiometrically documented hearing loss on at least one occasion
- Tinnitus or aural fullness in the treated ear
- Other cases excluded
- Certain Meniere’s disease
- Definite Meniere’s disease, plus histopathologic confirmation
| Staging of hearing loss in definite or certain Meniere’s is as follows: Stage: | Four Tone Average dB |
| 1 | <=25 |
| 2 | 26-40 |
| 3 | 41-70 |
| 4 | >70 |
The AAO-HNS also developed a functional level scale for use in surveys:
- Regarding my current state of overall function, not just during attacks (check the ONE that best applies):
- My dizziness has no effect on my activities at all.
- When I am dizzy I have to stop what I am doing for a while, but it soon passes and I can resume activities. I continue to work, drive, and engage in any activity I choose without restriction. I have not changed any plans or activities to accommodate my dizziness.
- When I am dizzy, I have to stop what I am doing for a while, but it does pass and I can resume activities. I continue to work, drive, and engage in most activities I choose, but I have had to change some plans and make some allowance for my dizziness.
- I am able to work, drive, travel, take care of a family, or engage in most essential activities, but I must exert a great deal of effort to do so. I must constantly make adjustments in my activities and budge my energies. I am barely making it.
- I am unable to work, drive, or take care of a family. I am unable to do most of the active things that I used to. Even essential activities must be limited. I am disabled.
- I have been disabled for 1 year or longer and/or I receive compensation (money) because of my dizziness or balance problem.
For reporting the results of treatment, the post-treatment meniere’s spells as a percentage of pre-treatment spells is used:
- 0 is Class A
- 1-40 is Class B
- 41-80 is Class C
- 81-120 is Class D
- >120 is Class E
Need to initiate secondary treatment is Class F.
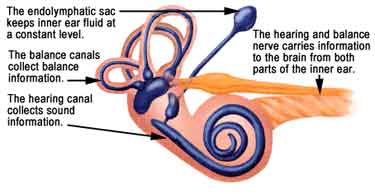
The physiology of the inner ear is intricately designed to allow hearing and balance. The perilymph, which exists outside of the membranous labyrinth, is similar in composition to CSF. It contains high sodium and low potassium content. The endolymph is similar in composition to intracellular fluid. It is low in sodium and high in potassium. Endolymph is believed to be produced by the stria vascularis or the membranous labyrinth. The membranous labyrinth separates endolymph from perilymph. While there is no difference in pressure between the two regions, there is a difference in charge of 80 mV.
There are several theories about the production and flow of endolymph, as put forward in a review article by James in 2004:
- Longitudinal – endolymph is produced in membranous labyrinth, flows to endolymphatic sac, then to dural venous sinuses
- Diffuse – endolymph is produced and absorbed along the membranous labyrinth
- Periodic Flow – endolymph flows only with changes in volume or pressure
Endolymphatic hydrops leads to distortion of the membranous labyrinth. A build up in pressure may lead to micro-ruptures of the membranous labyrinth. Minor et al posit in their 2004 review article that this build-up in pressure may lead to microruptures of the membranous labyrinth. The intermittent ruptures may be responsible for the intermittent nature of the attacks. Healing of the ruptures may account for return of hearing.
The etiologic agent for hydrops is not clear. Endolymphatic sac or duct obstruction has been proposed as an etiology. Though animal models in which hydrops is induced by endolymphatic sac obstruction, not all animals exhibit this effect and vertigo/nystagmus is present in few of those animals with hydrops. Clear a poorly understood alteration of production or absorption of endolymph is the cause of hydrops. Although immunologic insult to the inner ear has been proposed as an inciting event, this theory is controversial. Additionally, the role of hydrops itself in causation of Meniere’s is not clear. Rauche et al in 1998 performed a study of 19 temporal bones with hydrops and did chart reviews. Upon chart reviews, 13 patients had Meniere’s and 6 did not, suggesting that a subgroup of individuals have hydrops, but no Meniere’s.
The natural course of Meniere’s disease is often relenting. Silverstein et al in 1989 retrospectively reviewed patients with severe Meniere’s disease who refused surgery and found that 57-60% of patients had few or no Meniere’s-type complaints at 2 years, and 71% had few or no complaints at 8 years. The long term pure tone average in the group was about 50dB, with a 53% speech discrimination score. Caloric response was reduced 50%.
Medical management of Meniere’s disease can be grouped into two categories: acute treatment and maintenance therapy. There is little controversy over medications to use for acute vertiginous symptoms. Medications with anticholinegrnic, antihistaminergic, and antiemetic properties are useful. See Slide #16 in the PowerPoint presentation for a table comparing some available acute remedies.
Maintenance, or preventive medical therapy is much more controversial. Diuretics and salt restriction are often cited as the first-line treatment for Meniere’s disease. The putative mechanism of action is to alter fluid balance in the inner ear leading to a depletion of endolymph. Shinkawa and Kimura, in 1986 animal studies, were unable to demonstrate any beneficial effect on hydrops. Ruckenstein et al (1991) evaluated data from two double-blind studies by Klockhoff and Linblom and found that there was no statistical difference in measures of hearing, tinnitus, vertigo, or general condition between placebo groups and groups receiving diuretics.
Osmotic Diuretics such as urea or glycerol have been consistently shown to reduce symptoms in patients with Meniere’s, but the effect lasts only for a few hours. Objective data about the efficacy of osmotic diuretics includes the normalization of the SP:AP ratio on electrocochleography.
Acetazolamide is a diuretic that has has been shown to increase symptoms and hearing loss when given IV. It showed no benefit when given by mouth.
Vasodilators are purported to work by decreasing ischemia in the inner ear and allowing better metabolism of endolymph. Betahistine, a histamine agonist, has been a popular choice, albeit, not an intuitive one because antihistamines are used to combat acute symptoms. While several studies have claimed to show decreased vertigo with use of betahistine, a comprehensive review of the literature in Cochrane Database (2004) by James, et al found only one grade B study and four grade C studies, none of which produced convincing evidence for use of betahistine.
Immunologic therapy has been attempted for management of Meniere’s. Systemic and intratympanic steroids have been of questionable efficacy. A double-blinded prospective crossover study bye Silverstein et al showed no difference from placebo with intratympanic dexamethasone injections in patients with severe disease. He posited that steroids may have some efficacy in milder disease.
The Meniett Device, by Xomed, is an FDA approved class II device used for treatment of vertigo. The advocates of the device do not present a strong case for why it should work. It is a portable, low intensity, alternating pressure generator that is applied to the external auditory canal. It transmits pressure to the round window via a tympanostomy tube. Gates et al in 2004 published a prospective, randomized, placebo controlled trial of the Meniett device. Gates is also a paid consultant of Xomed. The study showed a statistically significant difference in “vertigo scores” between 1 and 3 months, with the users of the device reporting better control of symptoms. The difference vanishes at four months. The study was a short-term one (2 year data is pending) and did not use standardized measures of vertigo. Also good data on objective testing was not provided.
Intratympanic therapies aim to maximize the local effects of medication in the inner ear while minimizing systemic effects. The round window is the point of diffusion to the inner ear, and so some authors recommend visualizing the round window and removing mucosal bands that are often present over it. Aminoglycoside antibiotics, particularly gentamicin, are the most commonly applied intratympanic therapies. They damage hair cells of the crista, ampulla and cochlea.
Fowler in 1948 and later Schuknecht established the role of systemic streptomycin for bilateral disease, given 2g intravenously every day until bedside findings such as nystagmus, unsteadiness, or hearing loss were noted. Hearing loss and oscillopsia were a problem with this therapy, though reduction of the dosage of medications seemed to help. Systemic aminoglycoside administration is rarely indicated.
Many methods of intratympanic delivery of gentamicin exist. Side effects for all of the delivery methods include temporary imbalance or nystagmus, and hearing loss. Titration therapy is a well-established and popular regimen that was studied again recently by Martin and Perez in 2003. The prospective study of 71 subjects with severe vertigo is summarized below:
- Serial daily injections of buffered (pH 6.4) 26.7mg/cc gentamicin solution via 27 gauge needle into middle ear
- Injections repeated until vestibular symptoms developed (spontaneous or evoked nystagmus)
- At 2 years, 69% had Class A vertigo control, 14.1% had Class B
- 32.4% had hearing loss
The study overall shows a high rate of good responders, at 83.1%. But hearing loss was high, as is a problem with many gentamicin therapies.
Another method of gentamicin therapy is ablation using multiple daily treatments. A study by Jackson and Silverstein of 92 patients treated over an eight month period explores this method:
- Jackson and Silverstein – Study on 92 patients who underwent myringotomy and wick placement through to round window niche.
- Pts. self-administered gentamicin drops TID until 100% reduction on ENG of vestibular response
- 85% relief of vertigo, 67% improvement in aural pressure
- 36% hearing loss
Harner et al in 2001 advocated low dose therapy:
- Harner et al 2001 – retrospective study of 51 patients who received 1 dose of 40mg/mL injection and were re-evaluated in 1 month and given another if needed
- At 2 years, 86% had vertigo class A or B
- He reported minimal change in PTA but drop in SRT’s
- Claimed better hearing preservation with this regimen
Another method of gentamicin delivery is weekly administration of a single dose of gentamicin treatment for four treatments, or a continuous administration via microcatheter delivery. The microcatheter method results in exetremely variable total dosage of gentamicin.
Chia et al performed a meta-analysis of different modalities of therapy in 2004. They found that low-dose therapy was the least effective in controlling symptoms, which is not surprising because of the lower amount of gentamicin used. However, hearing preservation was no better in this group than any other. The titration method exhibited the best results, and had the best hearing outcomes. Hearing loss was greatest for multiple daily dosing, but vertigo symptoms were not more improved in this group. Chia recommended titration therapy as a very useful method.
Endolymphatic sac surgery is purported to address the site of obstruction causing hydrops. There are 4 basic types of endolymphatic sac surgery:
- Decompression – removal of bone around the sac
- Shunting – placement of synthetic shunt to drain endolymph into mastoid
- Drainage – incision of the sac to allow drainage
- Removal of sac – to address the possibility that the sac may actually play a role in endolymph production
Jens Thomsen et al (1981) performed a double-blinded placebo-controlled study comparing a sham surgery (cortical mastoidectomy) to endolymphatic shunt placement in 30 patients. Though all patients (placebo and control) statistically improved after surgery, there was no difference between placebo and control groups. A previously mentioned study by Silverstein in patients who refused surgery showed that non-operated patients did as well as operated patients. Endolymphatic sac surgery remains an extremely controversial operation. Potential complications include CSF leak, damage to the posterior semicircular canal, and meningitis.
Vestibular nerve section has been advocated because it can achieve vestibular suppression with minimal effect on hearing. It is a single step procedure, but often requires a neurosurgical approach (middle fossa, retrolabyrinthine/retrosigmoid) with the attendant potential complications of damage to the facial nerve, cochlear nerve, CSF leak, and meningitis. Hillman et al in 2004 retrospectively compared vestibular nerve sectioning to weekly intratympanic gentamicin. They showed significantly better vertigo control rates (25/27 Vertigo class A or B, 2.9 point improvement in functional level scale) compared to IT gent (10/15 class A or B, 2.3 point improvement in functional level). Hearing preservation was dramatically better in the vestibular nerve sectioning group (see slide 40 of powerpoint presentation). In spite of these seemingly superior results, many patients either cannot, or will not want to undero and intracranial procedure when a minimally invasive one with good results exists. Hillman et al reported a 12.6% incidence of CSF leak requiring lumbar puncture and extended hospital stay.
Another surgery for Meniere’s disease is the labyrinthectomy, which can be done through the mastoid or transcanal. This procedure is useful in patients with no serviceable hearing or who cannot tolerate an intracranial procedure. It is similar in efficacy to vestibular nerve section.
A bewildering array of medical and surgical therapies exist for treatment of Meniere’s disease. The therapies that are well-accepted and likely beneficial include vestibular suppressant medications, intratympanic gentamicin, vestibular nerve section and labyrinthectomy. Though the other treatments have some strong advocates, they are clouded in controversy.
Eustachian Tube
The eustachian tube (pharyngotympanic tube) connects the middle ear cavity with the nasopharynx. It aerates the middle ear system and clears mucus from the middle ear into the nasopharynx. Opening and closing functions of the eustachian tube are physiologically and pathologically important. Normal opening of the eustachian tube equalizes atmospheric pressure in the middle ear; closing of the eustachian tube protects the middle ear from unwanted pressure fluctuations and loud sounds. Mucociliary clearance drains mucus away from the middle ear into the nasopharynx, thus preventing infection from ascending to the middle ear.
Abnormal or impaired eustachian tube functions (ie, impaired opening or closing, defective mucociliary clearance) may cause pathological changes in the middle ear. This in turn can lead to hearing loss and other complications of otitis media. These pathological changes include recurrent acute otitis media and otitis media with effusion. Chronic retraction of the tympanic membrane may also lead to middle ear atelectasis and subsequent adhesive otitis media. A retraction pocket of the tympanic membrane secondary to chronic eustachian tube dysfunction may eventually evolve into cholesteatoma and potentially serious complications.
The eustachian tube lumen develops in the embryo by the lateral extension of the endoderm of the first pharyngeal pouch as it touches the inner surface of the ectoderm of the first branchial cleft. During this process, the distal portion of the pouch expands, forming the tubotympanic recess, which will later evolve into the middle ear cavity. The proximal portion narrows to form the eustachian tube. This process takes place during the first 10 weeks of gestation.
The cartilage and muscles of the eustachian tube develop from the surrounding mesoderm during the ensuing weeks. The levator veli palatini and the tensor veli palatini muscles seem to develop earlier than the cartilage and glandular tissue.
The cartilaginous portion of the tube elongates during the middle and third trimester until it reaches approximately 13 mm in length at term. Other morphologic changes also occur during that time with further development of the glandular structures and folding of the epithelium.
As the skull base grows down, the angle of the eustachian tube changes gradually from horizontal to oblique. This process continues after birth and well into adulthood.
ANATOMY OF THE EUSTACHIAN TUBE
Cartilaginous and bony framework
The eustachian tube in the adult is approximately 36 mm long and is directed downward, forward, and medially from the middle ear. It consists of 2 portions, a lateral third (12 mm), which is a bony portion arising from the anterior wall of the tympanic cavity, and a medial two thirds (24 mm), which is a fibrocartilaginous portion entering the nasopharynx. The tube opens about 1.25 cm behind and slightly below the posterior end of the inferior turbinate.
The bony portion is widest at its tympanic end. It passes through the squamous and petrous portions of the temporal bone but narrows gradually to the isthmus, which is the narrowest part of the eustachian tube.
The cartilaginous portion consists of a plate of cartilage posteromedially. The cartilage bends forward to form a short flange. The rest of the anterolateral wall is formed by fibrous tissue. The apex or lateral end of the cartilaginous part joins the bony portion at the isthmus; the wider medial end lies under the mucosa of the nasopharynx and raises the mucosa to form the tubal elevation (torus tubarius). Just behind this elevation is a recess called the fossa of Rosenmüller, which is a common site of origin for nasopharyngeal carcinoma and occult primary tumors.
The cartilaginous eustachian tube is attached to the skull base in a groove between the petrous part of the temporal bone and the greater wing of the sphenoid.
The lumen of the eustachian tube is roughly triangular, measuring 2-3 mm vertically and 3-4 mm horizontally. The bony portion is always open; the fibrocartilaginous portion is closed at rest and opens only on swallowing, yawning, or forceful inflation.
The eustachian tube in infants measures approximately 18 mm in length. It is about half the size of the adult eustachian tube and is generally more horizontal and less angulated. The bony portion is relatively longer and wider in diameter, the nasopharyngeal end of the cartilaginous portion lies more inferiorly.
At its nasopharyngeal opening, the eustachian tube is lined by respiratory epithelium that includes columnar ciliated cells, goblet cells, and mucous glands. The respiratory epithelium blends with the middle ear mucosa in the bony portion of the tube.
Ostmann fat pad
This fat pad is located in the inferolateral aspect of the eustachian tube and is thought to be an important contributing factor in closing the tube. It is also quite likely to contribute in the protection of the eustachian tube and the middle ear from retrograde flow of nasopharyngeal secretions.
RELATIONS OF THE EUSTACHIAN TUBE
Muscles
The muscles of the eustachian tube system help open and close the tube, thus allowing it to perform its function. These muscles are the (1) tensor veli palatini, (2) levator veli palatini, (3) salpingopharyngeus, and (4) tensor tympani.
The tensor veli palatini muscle originates from the bony wall of the scaphoid fossa and from the whole length of the short cartilaginous flange that forms the upper portion of the front wall of the cartilaginous tube. The muscle runs downward, converging into a short tendon that turns medially around the pterygoid hamulus. It then fans out within the soft palate and mingles with the fibers from the opposite side in the midline raphe. The tensor veli palatini separates the eustachian tube from the otic ganglion, the mandibular nerve and its branches, the chorda tympani, and the middle meningeal artery.
The salpingopharyngeus is a delicate muscle that is attached to the pharyngeal end of the eustachian tube and blends with the palatopharyngeus muscle downward.
The levator veli palatini has 2 origins: the lower surface of the cartilaginous tube and the lower surface of the petrous bone. At first, the levator is inferior to the tube; it then crosses to the medial side and merges into the soft palate.
Blood vessels
The arterial supply of the eustachian tube is derived from the ascending pharyngeal and middle meningeal arteries. The venous drainage is carried to the pharyngeal and pterygoid plexus of veins. The lymphatics drain into the retropharyngeal lymph nodes.
Nerves
The pharyngeal branch of the sphenopalatine ganglion derived from the maxillary nerve (V2) supplies the ostium. The nervus spinosus derived from the mandibular nerve (V3) supplies the cartilaginous part, and the tympanic plexus derived from the glossopharyngeal nerve supplies the bony portion of the eustachian tube.
THREE PHYSIOLOGIC FUNCTIONS OF THE EUSTACHIAN TUBE:
The physiologic functions of the eustachian are:
- Ventilation or pressure regulation of the middle ear
- Protection of the middle ear from nasopharyngeal secretions and sound pressures
- Clearance or drainage of middle ear secretions into the nasopharynx
Ventilation or pressure regulation
The normal eustachian tube at rest is collapsed, with perhaps slight negative middle ear pressure. Repeated opening of the eustachian tube actively maintains normal atmospheric pressure.
The eustachian tube opens upon swallowing or yawning by contraction of the tensor veli palatini muscle. Defective tensor veli palatini muscle function in cleft palate results in eustachian tube dysfunction. The role of the levator veli palatini muscle is unclear. Its contribution in opening the eustachian tube has been questioned.
Eustachian tube ventilatory function is less efficient in children than in adults. In addition, repeated upper respiratory tract infections and enlarged adenoids in children further contribute to the increased incidence of middle ear disease in children. However, as children grow, eustachian tube function improves as evidenced by the reduced frequency of otitis media from infancy to maturity.
Normally, the eustachian tube opens frequently, stably maintaining the middle ear pressure between +50 mm and -50 mm H2O. However, pressures above and below this range do not necessarily indicate middle ear disease.
About 1 mL of air or gas may be absorbed from the middle ear in 24 hours. The mastoid cell system is thought to function as a gas reservoir for the middle ear.
Protection
The eustachian tube is closed at rest. Sudden loud sounds are thus dampened before reaching the middle ear through the nasopharynx.
Patulous eustachian tube is an abnormal but not uncommon condition in which the tube is abnormally patent. The patient often complains about echoing when he or she talks (autophony), as well as ear fullness. Rapid weight loss may lead to decreased size of the Ostmann fat pad, which is thought to contribute to this condition.
The eustachian tube drains normal secretions of the middle ear by the mucociliary transport system and by repeated active tubal opening and closing, which allows secretions to drain into the nasopharynx.
A derangement in the closed middle ear system, such as tympanic membrane perforation or after mastoid surgery, sometimes results in reflux of nasopharyngeal secretions into the tube and can cause otorrhea. Similarly, forceful nose blowing creates high nasopharyngeal pressure and may force nasopharyngeal secretions into the middle ear.
Conversely, a relative negative middle ear pressure, as occurs in aircraft or scuba diving descent, may lock the eustachian tube. This leads to stagnation of secretions, and effusion collects in the middle ear as otitic barotrauma evolves. Inflation of the eustachian tube by the Valsalva maneuver or by politzerization can break the negative pressure in the middle ear and clears the effusion.
The middle ear is also protected by the local immunologic defense of the respiratory epithelium of the eustachian tube, as well as its mucociliary defense (clearance). A pulmonary immunoreactive surfactant protein has been isolated from the middle ears of animals and humans. It is thought to have the same protective function in the middle ear.
Clearance or drainage
Drainage of secretions and occasional foreign material from the middle ear is achieved by the mucociliary system of the eustachian tube and the middle-ear mucosa and muscular clearance of the eustachian tube, as well as surface tension within the tube lumen.
The flask model proposed by Bluestone and his colleagues helps to better explain the role of the anatomic configuration of the eustachian tube in the protection and drainage of the middle ear. In this model, the eustachian tube and middle ear system is likened to a flask with a long narrow neck. The mouth of the flask represents the nasopharyngeal end, the narrow neck represents the isthmus, and the middle ear and mastoid gas cell system represents the body of the flask. Fluid flow through the neck depends on the pressure at either end, the radius and length of the neck, and the viscosity of the liquid. When a small amount of liquid is instilled into the mouth of the flask, the liquid flow stops somewhere in the narrow neck due to the narrow diameter of the neck and the relative positive air pressure in the chamber of the flask. However, this does not take into consideration the dynamic role of the tensor veli palatini muscle in actively opening the nasopharyngeal orifice of the eustachian tube.
EUSTACHIAN TUBE FUNCTION TESTS
A functional and patent eustachian tube is necessary for ideal middle ear sound mechanics. A fully patent eustachian tube may not necessarily have perfect functioning, as is the case with the patulous eustachian tube or with mucociliary abnormalities. Testing of both eustachian tube patency and function are therefore important.
Pneumatic otoscopy
Permeatal examination of the tympanic membrane assesses the patency and perhaps the function of the tube. A normal appearing tympanic membrane usually indicates a normally functioning eustachian tube, although this does not preclude the possibility of a patulous tube.
Otoscopic evidence of tympanic membrane retraction or fluid in the middle ear indicates eustachian tube dysfunction but cannot be used to differentiate between functional impairment and mechanical obstruction of the tube. Normal tympanic membrane mobility on pneumatic otoscopy (siegalization) indicates good patency of the eustachian tube.
Nasopharyngoscopy
Nasopharyngoscopy by posterior rhinoscopic mirror examination or more accurately by fiberoptic endoscope helps visualization of any mass (eg, adenoids, soft tissue growth in the nasopharynx) that may be obstructing the pharyngeal end of the eustachian tube.
Attempts have been made to assess eustachian tube function with the help of nasopharyngoscopy. Yagi and colleagues evaluated the patency of the eustachian tube using a fiberoptic endoscope and a photoelectric device (phototubometry). Using videoendoscopy of the ear, Poe and colleagues assessed tubal function in adults and observed various disease processes such as inflammation of the tube and patulous dysfunction. This method has been gaining popularity in the assessment of patients suspected to have eustachian tube dysfunction.
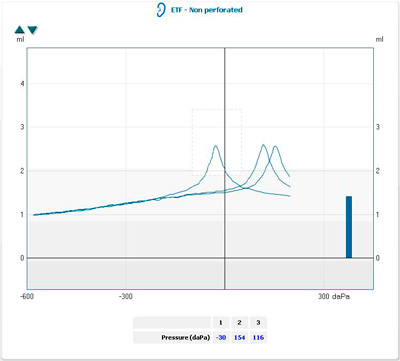
Tympanometry
Measuring middle ear pressure with an electroacoustic impedance meter helps to assess eustachian tube function.
High negative middle ear pressure (> -100 daPa) indicates eustachian tube dysfunction. High negative pressures may be seen in individuals with normal hearing; however, a nearly normal middle ear pressure may be associated with hearing loss.
In the presence of tympanic membrane perforation, the air passes into the middle ear resulting in a large canal volume on tympanometry.
Imaging
With the recent development of advanced imaging technology, studies have been used to better define the anatomy and pathology of the eustachian tube. MRI has been used to visualize the eustachian tube and to assess its anatomy and pathology in patients with nasopharyngeal carcinoma. Moreover, MRI has been used in experimental animal models to evaluate middle ear inflammation. It has more accurately been also used to assess the effect of experimentally induced functional obstruction of the eustachian tube by botulinum toxin A on the middle ear.
CT has also been used to assess the tube in normal individuals, in patients with patulous eustachian tube, and in otitis media. It has also been used in studying eustachian tube clearance. Fluoroscopy with contrast provides dynamic evaluation of mucociliary clearance.
Eustachian tube catheterization
Catheterization of the eustachian tube with a curved metal cannula via the transnasal approach has been used to assess tubal function for more than 100 years. It can be done blindly, with the help of a nasopharyngoscope, or transorally with a 90° telescope.
The catheter is passed along the floor of the nose until it touches the posterior wall of the nasopharynx. The catheter is then rotated 90° medially and pulled forward until it impinges on the posterior free part of the nasal septum. The catheter is then rotated 180° laterally, so that its tip lies at the nasopharyngeal opening of the eustachian tube. A Politzer bag is attached to the outer end of the catheter, and an auscultation tube with 2 ear tips is used with one tip in the patient’s ear and the other in the examiner’s ear. Air is pushed into the catheter by means of the Politzer bag. The examiner hears the rush of air as it passes through the catheter into the eustachian tube and then into the middle ear.
Successful transferring of applied positive pressure from the proximal end of the cannula into the middle ear suggests tubal patency. Normal blowing sounds mean a patent eustachian tube and bubbling indicates middle ear fluid. Whistling suggests partial eustachian tube obstruction while absence of sounds indicates complete obstruction or failed catheterization.
Valsalva and Politzer tests
In the Valsalva test, the eustachian tube and middle ear are inflated by a forced expiration with the mouth closed and the nose pinched by the thumb and forefinger. The effect of high positive nasopharyngeal pressures at the proximal end of the eustachian tube system can be evaluated qualitatively. When the tympanic membrane is intact, the overpressure in the middle ear can be observed by otoscopy as a bulging tympanic membrane. When the tympanic membrane is perforated, the sound of the air escaping from the middle ear can be heard with a stethoscope or with the Toynbee tube.
The Politzer test is similar to the Valsalva test, but instead of positive nasopharyngeal pressure being generated by the patient, the nasopharynx is passively inflated. This is achieved by compressing one nostril into which the end of a rubber tube attached to an air bag has been inserted while compressing the opposite nostril by finger pressure. The subject is asked to swallow or to elevate the soft palate by repeating the letter “k.”
Both the Valsalva and Politzer tests are outdated and rarely used clinically for assessment of eustachian tube function. These maneuvers may be more beneficial in the management of some patients. Nevertheless, the efficacy of these procedures for treatment of middle ear effusion is controversial, and they are not without potential risks. The author has encountered a case of meningitis following Politzerization for the treatment of otitis media with effusion in an otherwise healthy elderly man.
Toynbee test
This test is considered more reliable than the previous 2 in the assessment of eustachian tube function. On closed nose swallowing, negative middle ear pressure develops in healthy persons. In an intact tympanic membrane, pneumatic otoscopy or tympanography can be used to measure changes in middle ear compliance. In a perforated tympanic membrane, the manometer of the impedance bridge can be used to measure middle ear pressure changes
OTHER TESTS OF EUSTACHIAN TUBE FUNCTION
Other tests have been developed for assessment of eustachian tube function. A 9-step inflation-deflation tympanometric test is used to assess changes in resting middle ear pressure after applying positive and negative ear canal pressures while the patient is swallowing.
Other methods include microflow technique, impedance method, sonometry, forced response test, and videoendoscopy of the nasopharyngeal end of the eustachian tube.
A self-described method by patients with perforated tympanic membranes is the bitter taste of ear drops in the mouth when used topically. This indicates a patent eustachian tube.
A final testing mechanism is sonotubometery. The advantage of this diagnostic test is the ability to evaluate the eustachian tube function with or without an intact tympanic membrane under physiologic conditions.
Patulous Eustachian tube, also known as patent Eustachian tube, is the name of a rare physical disorder where the Eustachian tube, which is normally closed, instead stays intermittently open. As a result, when it is open, all of the patient’s breathing, talking, swallowing, heart beat, etc. vibrates directly on the ear drum creating an effect that sounds like the patient has a bucket on his/her head. The medical term for this phenomenon is autophony, the hearing of self-generated sounds PET is a physical disorder. The exact causes may vary depending on the person, however weight loss is a very common cause.The eustachian tube remains closed when normal most of the time, and there is fat tissue surrounding it that holds it closed. Weight loss, even as little as 5 lb (2 kg), may be enough to cause the tissue surrounding the Eustachian tube to shrink. This will cause it to remain open.
Caffeine, especially the amount in coffee, is also a major cause, as it may dehydrate enough to affect the surrounding tissue. Exercise does the same thing, although for a shorter amount of time.
VASOMOTOR RHINITIS
Vasomotor rhinitis is a non-infective, non-allergic condition characterised by profuse rhinorrhoea and sneezing, with or without nasal obstruction, occurring in attacks which may be either paroxysmal or parennial. The running of the nose and sneezing may be so severe as to even disable the patient.
Malcomson showed conclusively that stimulation of the parasympathetic or interruption of the sympathetic nerve supply to the nasal mucous membrane caused vasodilatation, hypersecretion and sneezing, and hence it is reasonable to assume that under normal conditions there exists a balance between the two systems. The symptoms of vasomotor rhinitis seem to be due to an imbalance between these two systems.
While the causes of vasomotor rhinitis could be psychogenic or due to drugs like methyldopa or rauwolfia, or endocrine, the logical step in the treatment of idiopathic nasal vasomotor imbalance is vidian neurectomy, when it is found that all other available methods of treatment have failed and the symptoms are severe enough to justify surgery.
The principle underlying vidian neurectomy is to sever the preganglionic fibres that reach the sphenopalatine ganglion through the vidian nerve. Several routes have been described to approach the deep seated vidian nerve: (1) Transantral (a) Classic,[2] (b) Subperiosteal,[7] (2) Transeptal,[5] (3) Transpalatal,[1],[6] (4) Transethmoidal,[9] and (5) Transnasal.[3],[8]
The multiplicity of these routes suggests that none of them is entirely satisfactory. This is because the vidian nerve is deep seated, at the base of the skull, behind the pterygopalatine fossa and lateral to the nose. This is an area which is anatomically difficult to reach and is surrounded by numerous important structures. We practise and advocate a direct transnasal approach[3] to the vidian nerve to effect a preganglionic section of the nerve. Having operated on 357 cases by this route, we are convinced that this is a simple and direct approach to the vidian nerve because of the special anatomical relationship between the sphenopalatine foramen on the lateral wall of the nose and the mouth of the vidian canal on the posterior wall of the pterygopalatine fossa. This paper deals with these anatomical considerations as applied to vidian neurectomy.
Material and Methods
The anatomy of the vidian canal, the sphenopalatine foramen, and the pterygopalatine fossa was studied with special reference to the transnasal route of approach to the vidian canal. This was accomplished by means of a study of 30 dry skulls sectioned at various levels in the sagittal, coronal and horizontal planes. This was supplemented by dissections of 30 fresh cadaver specimens to determine the gross anatomy of the region. The findings were further corroborated by observations made on 357 patients who underwent a transnasal vidian neurectomy.
Observations
The following description of anatomy of the pterygopalatine fossa, although well known, is included here because of its relevance to our observation of certain anatomical features in this region which are mentioned later. The pterygopalatine fossa is bounded anteriorly by the posterior surface of the maxilla and posteriorly by the anterior surface of the pterygoid process, which has two openings, namely, the foramen rotundum and the vidian canal [Fig. 1A]. Medially, it is bounded by the vertical plate of the palatine bone. This has a notch on its upper border which is bounded anteriorly by a larger orbital process and posteriorly by a short sphenoidal process [Fig. IA]. This sphenopalatine notch is converted into the sphenopalatine foramen by the under surface of the body of the sphenoid. It transmits the sphenopalatine artery and nerves from the pterygopalatine fossa into the nose and is the main focus of interest in the transnasal vidian neurectomy.
The vidian nerve enters the posterior opening of the vidian canal and traverses the canal situated on the floor of the sphenoid sinus where it often produces a ridge. The vidian canal is directed anteriorly and slightly laterally (about 5° inclination) and opens into the posterior wall of the pterygopalatine fossa. Its anterior opening is funnel shaped, and is 8-9 mm below and medial to the anterior opening of the foramen rotundum. Thus there is a distinct bony ridge between the two foramina.
After synapsing in the sphenopalatine ganglion, the postganglionic fibres are distributed to the mucous membrane of the nose, the palate, and the lacrimal gland.
We have observed that the sphenopalatine foramen and the mouth of the vidian canal have a very consistent and special anatomical relationship to each other. The sphenopalatine foramen and the funnel shaped mouth of the vidian canal are always situated in the same horizontal plane, the former being in the medial wall and the latter being in the posterior wall of the pterygopalatine fossa. They are separated only by a few millimetres of bone as seen in.
It has also been our observation that the sphenopalatine foramen is situated in level with, and posterior to the bony middle nasal turbinate. The posterior end of the bony attachment of the middle turbinate presents a sharp ridge of bone called the ethmoidal crest. We find that this crest is a very constant feature and is always located at the antero-inferior margin of the sphenopalatine foramen. This constancy of location makes it an invaluable guide in locating the sphenopalatine foramen during surgery. Having identified this foramen, one has only to advance a probe through it, hugging the medial wall of the pterygopalatine fossa, to reach the funnel shaped opening of the vidian canal. It may be stressed here that a probe so advanced through the sphenopalatine foramen will invariably enter the mouth of the vidian canal. This is the essence of the transnasal vidian neurectomy.
In the transantral operation, the maxillary antrum is entered from its anterior wall by a preliminary Caldwell-Luc operation. The posterior wall of the antrum is opened to reach the pterygopalatine fossa. To reach the vidian canal, one has to encounter and pass beyond the veins, arteries, nerves and fat in the pterygopalatine fossa, all of which make the operation very difficult.
In the direct transnasal operation, the approach to the vidian nerve is through the sphenopalatine foramen. The middle turbinate is lifted by a Killian’s speculum. The mucosa under the posterior end of the middle turbinate is incised and elevated to expose the ethmoidal crest. Postero-superior to this is the sphenopalatine foramen from which the sphenopalatine vessels may sometimes bleed. This bleeding is easily controlled by cauterization. A probe is passed through the sphenopalatine foramen and advanced postero-laterally till the anterior funnel shaped opening of the vidian canal is reached. Any further advancement of the probe is stopped by the lateral wall of the anterior opening of the vidian canal. The vidian nerve is then cauterized.
The success of the preganglionic section of the vidian nerve is confirmed by decreased lacrimation on the operated side as tested by the Schirmer’s test.
Discussion
The most popular approach to the vidian nerve is the transantral route. This, however, is fraught with certain difficulties viz. damage to the maxillary artery or nerve, a risk of ophthalmoplegia due to deep penetration by the probe, and other complications like infra-orbital anaesthesia and neuralgia and maxillary sinusitis.
The transpalatal route is cumbersome as it involves elevation of a palatal flap and bony dissection. Technical difficulties arise when extensions of the sphenoid sinus engulf the pterygoid canal. There is a risk of palatonasal fistula and injury to the internal carotid artery. In addition, there is a relatively long convalescence.
The transseptal approach involves a lot of stretching and manipulation of tissues with an added risk of septal perforation.
In the preganglionic section by the transnasal route, a direct approach to the vidian canal is possible as the sphenopalatine foramen and the funnel shaped anterior opening of the vidian canal are in the same horizontal plane. The identification of landmarks is easy due to the clear-cut ethmoidal crest and the presence of the sphenopalatine vessels which pinpoint the position of the sphenopalatine foramen. The contents of the pterygopalatine fossa are bypassed, thus precluding any damage to the mixillary artery, the maxillary nerve and the sphenopalatine ganglion. Even the fat in the fossa does not feature in the dissection. There is never a risk of the probe entering deep into the vidian canal due to obliquity in the directions of the probe and the vidian canal. Because of this obliquity, a rigid probe will never be able to enter weep into the vidian canal. In the transantral operation, on the other hand, the direction of the vidian canal and that of the probe are almost in the same straight line, and hence the risk of the probe going deep into the vidian canal. Cauterization with such a deep seated probe will lead to damage to the abducent nerve intracranially. This is the cause of ophthalmoplegia and diplopia due to sixth nerve paralysis which sometimes occurs as a complication of the transantral route due to spread of the cautery burn. The whole procedure of transnasal vidian neurectomy takes less than 15 minutes for a bilateral neurectomy. It is done as an outpatient procedure under local anaesthesia, no nasal tamponade being necessary post-operatively. Further, it can be repeated easily if there is a recurrence of symptoms.
In conclusion, we would like to state that the clarity of landmarks and the ease with which the vidian canal can be approached by a direct transnasal route via the sphenopalatine foramen make it the approach of choice for vidian neurectomy.
The maxillary sinus is opened, using Caldwell-Luc’s procedure; a Ushaped mucosal incision is made and lifted up, exposing the posterior bony wall as well as a posterior part of the medial bony wall of the antrum. After removing the bony wall, the periosteum covering the pterygopalatine fossa and the nasal mucosa (underside) are revealed. There is a crevice between these soft tissues. The maxillary surface of the sphenoid can be easily touched by a small periosteal elevator inserted into the crevis. The medial corner of the pterygopalatine fossa is pushed aside laterally with the covering periosteum. This exposes a part of the bony surface of the maxillary surface of the sphenoid. Go upward, touching on the maxillary surface until the elevator goes into the funnel-like opening of the vidian canal. The content of the vidian canal is electrocauterized and sectioned. It is important to expose the whole circumference of the bony opening of the vidian canal. This guarantees the complete section of the vidian nerve. The canal is plugged with a piece of gelatine sponge and bone wax. The mucosal flap is returned back.
This surgical procedure may be properly termed as “Transantral Subperiosteal Vidian Neurectomy.”
MEDICAL MANAGEMENT OF VESTIBULAR DISORDERS AND VESTIBULAR REHABILITATION
Introduction
There are three basic inputs – vision, proprioception, and the vestibular semicircular canals and otolith organs – that are used by the brain to provide ocular stability, gait control, and balance during active and passive body movements. A disorder of the vestibular system is a major disruptor of these critical functions and a significant source of spatial disorientation symptoms. A patient’s complaint of dizziness can be caused by a variety of factors including pre-syncopal lightheadedness, dysequilibrium, visual distortion and multi-sensory dysfunction and must be differentiated clinically from vertigo or dizziness of vestibular origin. The differential diagnosis of vertigo has remained stable over the past several decades, but management strategies continue to improve. The goal of this discussion is to review the most common types of vestibular disorders encountered by the otolaryngologist and discuss the medical management and rehabilitation therapy strategies currently at use.
Pathophysiology
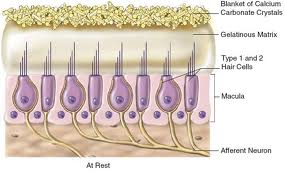
The vestibular labyrinth is responsible for detecting both linear and angular head movements. Each is composed of three semicircular canals (SCC) and two otolithic organs. The SCC detects rotational movement or angular accelerations and the otolith organs detect linear acceleration. The sensory hair cells within the membranous labyrinth detect specific movements during hyper of depolarization of the stereocilia and kinocilia extending from the cell body. Within the SCC, the hair cells are organized under a gelatin film called the cupula. In the utricle and saccule, the hair cells are attached to a layer of otoconia on the macula. The otoconia remain stationary relative to linear head accelerations, which causes deflection and stimulation of the underlying hair cells. The hair cells project one or more afferent nerve endings onto the body of the cell. In addition there can be a direct and indirect efferent innervation of the hair cell body. The fiber bundles from each of the five sensory epithelia join to make two branches of the vestibular nerve: the superior and inferior branches. The afferent nerve fibers are bipolar and have one synapse at the hair cell and the other in the vestibular nucleus. The cell bodies are located in between within Scarpa’s ganglion. Peripheral vestibular disorders are presumed to be restricted anatomically to the aforementioned structures.
In addition to a normally functioning vestibular system, balance requires input from the visual (vestibulo-ocular) and proprioceptive (vestibulospinal) systems. Vestibular input is balanced and compared to these inputs. Central causes of vestibular dysfunction compromise the central vestibular circuits that mediate vestibular influences on posture, control of gaze, and autonomic functions. Any insult that disrupts the calibration or balance between the two peripheral vestibular systems or between the vestibular system and its visual and proprioceptive input leads to the sensation of vertigo or loss of balance. If this process is acute, vertigo usually results. If it is more chronic, dysequilibrium may be the presenting symptom. The goal of treatment then becomes to restore balance between the input systems.
Also, the vestibular system influences or contributes to brainstem autonomic circuits. There is an intimate linkage in components of brainstem pathways that process vestibular and visceral inputs. This explains the autonomic dysfunction associated with alterations of vestibular input, including nausea, vomiting, and pallor, as well as changes in respiration and circulation.
Medical Treatment
The treatment of vertigo can be divided into to components: symptomatic and specific. Symptomatic treatment should focus on relieving the acute symptoms and autonomic complaints of vertigo. Specific treatments are geared towards targeting the underlying cause of the vertigo.
Symptomatic pharmacotherapy
There are several transmitters in the vestibular nuclei, including cholinergic, H1-histaminergic receptors, and GABA. The vomiting center is stimulated by serotonergic, dopaminergic (D2) and histaminergic (H1) systems. Thus, many pathways and neurotransmitters are responsible for vestibular symptoms and associated autonomic complaints. This explains why so many classes of drugs are used to manage acute vertigo attacks. The main classes of drugs used for symptoms of acute vertigo include antihistamines, anticholinergic agents, anti-dopaminergic agents, and (gamma)-aminobutyric acid-enhancing (GABA-ergic) agents. These drugs will not eliminate but rather reduce the severity of vertiginous symptoms.
Although the exact mechanism of action of these drugs is unclear, they act at the level of the neurotransmitters involved in the propagation of impulses from primary to secondary vestibular neurons and in the maintenance of tone in the vestibular nuclei. They also act on the areas of the nervous system that control vomiting, including the central components, or “emetic center” and the peripheral components of the gastrointestinal tract. Two recent clinical trials comparing IV dimenhydrinate (50mg) with lorazepam (2 mg) and IM dimenhydrinate (50mg) with droperidol (2.5mg) for the treatment of peripheral vertigo in patients in the emergency department, found that dimenhydrinate was more effective than lorazepam and that dimenhydrinate and droperidol were equally effective. The response is clearly dose-dependent, so higher doses can be tried if the initial dose is not effective. It is important to note that all the medications used can be sedating, so they should not be used in patients attempting to perform activities, which necessitate a high level of alertness (i.e., driving, operating machinery or athletics). Less sedating drugs, such as meclizine and transdermal scopolamine are useful for milder vertigo and prevention of motion sickness. The newer nonsedating antihistamines do not enter the CNS and have little value in the treatment of acute vertiginous attacks.
Specific Pharmacotherapy
Vestibular Neuritis
Characterized by the sudden onset of prolonged vertigo of peripheral origin usually without hearing loss, vestibular neuritis (a.k.a. vestibular neuronitis) typically exhibits severe vertigo over a period of hours, lasting a few days, and then subsiding over the course of a few weeks. It is thought to result from a selective inflammation of the vestibular nerve, presumably of viral origin. The onset may be preceded by a viral infection of the respiratory or gastrointestinal systems. This finding and the occurrence of epidemics, several family members afflicted, and more common spring and early summer disease all support a potential viral etiology. Therefore, treatment aimed at stopping the inflammation has been proposed. Ariyasu et al treated 20 patients in a double-blinded, crossover study with methylprednisolone versus placebo for acute vestibular vertigo. They found that 90% experienced a decrease in vertigo within 24 hours, compared with only 30% of the placebo group. The placebo groups were switched to methylprednisolone after 24 hours and subsequently had a decrease in vertigo within 24 hours of starting the steroid. The electro-nystagmogram in the 16 patients who took methylprednisolone with resolution of vertigo returned to normal within 1 month.
Most patients will have spontaneous, complete symptomatic recovery even only with supportive treatment. Patients who have persistent unsteadiness or motion provoked symptoms may have incomplete central compensation and should benefit from a customized vestibular rehabilitation program.
Meniere’s Disease
The treatment for Meniere’s disease or endolymphatic hydrops continues to evade clinicians, because the precise etiology of the disease is unknown. The histiologic finding of hydrops by Hallpike and Cairns in 1938 implicated a disturbance of salt and water balance as the pathology in patients suffering from Meniere’s. The widely accepted medical treatment has, therefore, been dietary salt restriction and diuretics. It is thought that diuretics can alter the fluid balance in the inner ear leading to a decrease in endolymph and resolution of the hydrops. Thiazide diuretics are traditionally used nowadays in combination with a potassium sparing diuretic such as triamterene (Maxzide or Dyazide). It is important to stay on the diuretic therapy for at least 3 months before a decision is made to discontinue therapy. If allergies to sulfa drugs contradict the use of thiazides, loop diuretics or other therapies may be considered.
Carbonic anhydrase inhibitors (e.g., acetazolamide), which have been used by ophthalmologists to decrease intraocular pressure in treating glaucoma, have been tried for Meniere’s. The analogy drawn between the two disease states (Meniere’s has been called “inner ear glaucoma”) led to the trial of these agents. These agents decrease sodium-hydrogen exchange in the renal tubule leading to diuresis, however the diuretic effects are not as long lasting as thiazides and loop-diuretics. In addition, acetazolamide is capable of decreasing CSF production. Possible adverse effects include nephrocalcinosis, mild metabolic acidosis and GI disturbances with chronic therapy.
Vasodilators have also been used for the treatment of Meniere’s disease, based on the hypothesis that the pathogenesis of endolymphatic hydrops results from ischemia of the stria vascularis. The rationale is to improve the metabolic function of a diseased ear. IV histamine, isosorbide dinitrate, cinnarizine (calcium antagonist) and betahistine (oral histamine analogue) have all been used with anecdotal success, but no studies have demonstrated definitive beneficial effects of vasodilator therapy in reversing hydrops.
It has recently become apparent that Meniere’s disease may be a disease of multifactorial inheritance. In some patient’s there is thought to be an association of immune-mediated phenomena. There have been numerous reports of association of allergies and Meniere’s disease. A study by Gottschlich et al., found that fifty percent of subjects meeting the criteria for Meniere’s were found to have antibodies to a 70-kD heat-shock protein, which has been implicated in autoimmune sensorineural hearing loss. Therefore, treatment with immunosuppressive agents has gained favor, especially for patients presenting with bilateral disease. Systemic and intratympanic glucocorticoids, cyclophosphamide, and methotrexate have all been used by clinicians. Shea reported 48 patients following dexamethasone transtympanic perfusion for intractable Meniere’s disease with a 66.7% success rate for the elimination of vertigo and a 35.4% improvement in hearing.
For intractable disease with disabling vertigo despite medical treatment, vestibular surgery should be considered. The chemical labyrinthectomy, or transtympanic gentamicin (intratympanic aminoglycoside [ITAG]), allows treatment of unilateral disease without producing systemic toxicity or affecting the opposite ear. It also carries the advantage of being a nonsurgical office procedure. Gentamicin is primarily vestibulotoxic, and may impair the function of vestibular dark cells, which are thought to play a role in the production of endolymph. However, there is an inherent risk of hearing loss that may be sudden, severe, and irreversible, which averages around 30%.
A stock solution of 40mg/mL of gentamicin is used and 10mg to 20mg is injected over the round window after anesthetizing the tympanic membrane. The patient remains in the supine position with the injected ear up for 30 minutes and is encouraged not to swallow. Bolus injections are typically repeated either weekly or biweekly, although the end point of therapy is variable between different authors, some advocating only one treatment and a possible follow-up. Clinical signs of vestibular hypofunction such as spontaneous nystagmus or headshake nystagmus are monitored in follow-up visits and considered the endpoint of therapy. Monitoring with audiometry between injections to detect potential hearing loss is advised. Total ablation of vestibular function does not appear necessary for this method to be effective because all studies report excellent control of vertigo. Minor was able to control vertigo in 91% of his treatment group with a 3% rate of profound SNHL. A recurrence rate of 22% occurred with control in all but one by further ITAG treatments. These numbers are very similar to vestibular neurectomy results with fewer risks. Continuous delivery methods involve a device that delivers the drug directly to the round window niche (Microwick, Round Window Microcatheter). Direct injection via labyrinthotomy has been tried with significant hearing loss and has fallen out of favor.
Benign Paroxysmal Positional Vertigo (BPPV)
BPPV is the most common cause of vertigo and results from dysfunction of the posterior semicircular canal (PSCC). Two theories have developed to explain its pathophysiology: cupulolithiasis and canalithiasis. The cupulolithiasis theory proposes that calcium deposits become embedded on the cupula, rendering the PSCC dependent on gravity. In the canalithiasis theory, calcium debris (displace otoconia) becomes displaced within the PSCC but does not adhere to the cupula. In any case, head movements, particularly looking up, lying down, or rolling over onto the affected ear, result in displacement of the canal “sludge” and resultant symptoms. Several approaches have been developed to treat BPPV, including particle repositioning maneuvers and habituation exercises.
Semont et al adhering to the cupulolithiasis theory proposed a liberatory maneuver as a single treatment alternative. The patient is asked to sit on the side of an examination table with the head turned away from the affected ear. The patient is then quickly moved to the lateral decubitus position, with the head facing the ceiling, and is kept there for 2 to 3 minutes. Then, the patient is moved rapidly through the sitting position and onto the opposite side with the head remaining in the original position (now facing the floor). After a few minutes, the patient is returned to the seated position and is asked to remain upright for 48 hours. The reported cure rates are 84% after one, and 93% following two treatments.
Epley favored the canalithiasis theory and proposed a canalith repositioning procedure inducing gravity-directed movement of particulate matter into the vestibule, where it should not produce symptoms. The patient is initially placed in the Dix-Hallpike position for the affected ear. The head is then slowly taken through extension to the opposite Dix-Hallpike after 2 to 3 minutes. After an equal interval of time in this position, the patient is rolled onto the unaffected shoulder with the head turned toward the floor. After another 2 to 3 minutes, the patient is returned to the seated position. Again, the patient is asked to remain upright for 48 hours after the procedure. Epley reported 80% cure after one treatment and 100% improvement after multiple sessions in 30 patients.
Brandt and Daroff designed habituation exercises requiring the patient to move into the provoking position repeatedly, several times a day. From the seated position, the patient first moves rapidly into the provocative position. After the vertigo stops, the patient returns to the upright, seated position for 30 seconds, then onto the opposite side for 30 additional seconds. This is repeated until the vertigo diminishes. The exercises should be performed every 3 hours until the vertigo resolves for 2 consecutive days. They report a 98% success rate after 3 to 14 days of exercises.
Blakley compared the canalith repositioning maneuver with no treatment, and found 89% of all patients were improved after 1 month with no statistical significance between the 2 groups. The timing of spontaneous remission showed a 50% cure rate after 1 month. Still, advocates of noninvasive treatment techniques claim results superior to these numbers.
Otosyphilis
Penicillin has been the established treatment of otosyphilis. Intramuscular and intravenous routes are both acceptable. 2.4 million units of benzathine penicillin IM weekly for three consecutive weeks is considered minimal treatment and some would argue that treatment should be extended for one year. If the IV route is chosen, 10 million units of penicillin G per day is given in divided doses for ten days, followed by 2.4 million units of IM benzathine penicillin per week x2. Probenecid increases the half-life and CSF penetration of penicillin and may improve these regimens. Penallergic patients can be dosed with 500mg of tetracycline or erythromycin qid for 30 days. Steroids in addition to penicillin has been shown to improve treatment.
Vertebrobasilar insufficiency (VBI)
VBI is characterized by vertigo, diplopia, dysarthria, gait ataxia and bilateral sensory and motor disturbance. Symptoms of transient ischemia are alarming but generally benign as there is rich collateral blood supply and a relatively low incidence of stroke. Antiplatelet therapy is warranted usually with aspirin. Ticlid, a platelet aggregate inhibitor, has also been used in these patients, but because of the risk of life-threatening neurtropenia, it is only warranted in those patients unable to tolerate aspirin.
Migraine
Many patients who suffer from migraine have concomitant vertigo and disequilibrium. Furthermore, if their headaches are controlled, they are often asymptomatic from their vertigo. Diagnostic criteria include personal or family history of migraines, motion intolerance, and vestibular symptoms that do not fit other vestibular disorders. Several theories are currently postulated as the mechanism underlying migraine. Symptoms may be of vascular origin, abnormal neural activity in the brainstem, or abnormal voltage-gated calcium channel genes. Treatment includes modifying risk factors, abortive medical therapy, and prophylaxis. These patients should avoid nicotine products, exogenous estrogens, and foods that exacerbate symptoms (red wine, sharp cheese, chocolate, MSG, etc.). Exercise programs and stress reduction are also important. Ergots, sumatriptin, and midrin are helpful in aborting acute attacks. Prophylactic medical therapy can be started if migraines occur several times a month (aspirin, ibuprofen, lithium, calcium channel blockers, amitryptiline and beta blockers).
Vestibular Rehabilitation Therapy (VRT)
The use of exercise in the rehabilitation of patients with vestibular disorders is aimed at promoting vestibular compensation. Many treatment approaches have been designed to promote compensation through habituation. Other approaches are designed to enhance the adaptation of the vestibulo-ocular and vestibulo-spinal reflexes so that less input is required from the vestibular system. Many of these exercises may initially exacerbate the patient’s symptoms, so counseling the patient to relieve anxiety and mistrust is important.
Cawthorne-Cooksey exercises were developed in the 1940s and include movements of the head, tasks requiring coordination of the eyes with the head, total body movements, and balance tasks. They recommended performing exercises at tolerable speeds, different positions, and with the eyes open and closed. Also, they had patients perform the exercises in loud and noisy environments, which is often more difficult in patients with vestibulopathies. There are many modified versions of this treatment philosophy in use today.
Candidates for rehabilitation therapy are those with stable lesions of the peripheral or central vestibular system, poor central integration, or abnormal motor function. Many elderly patients with multiple sensorimotor and musculoskeletal deficits fall into this category. The basis for exercise therapy assumes that central compensation following vestibular injury has occurred in an incomplete or inappropriate manner, the patient’s symptoms stem from this incorrect compensation and not from fluctuating disease, and customized therapy is more effective than general exercise in changing this compensatory status.
The current common techniques of VRT still include habituation of pathologic responses, postural control exercises, visual-vestibular interaction, and conditioning activities. While standardized exercises are helpful, therapists can individualize treatments by first identifying the pathologic movement that causes the symptoms and developing a list of activities that reproduce the movements. Those movements can be incorporated into normal daily activities so they are reliable and remain interesting to the patient. The exercises should be performed twice daily unless limited by nausea and vomiting. Many patients report improvement within 4 to 6 weeks. However, the longer the problem has existed, the longer the recovery time will be.
Gait exercises can also be added to improve postural control. The starting point may be simple static balance exercises with eyes open and closed, on a stable support surface or on foam. The base of support can be gradually decreased to stress the patient’s balance. Practice generally improves overall postural control. The patient also needs the practice of walking in different environments (grass, malls, or at night).
An important point to remember is that the input from two sensory systems is required for adequate postural stability. If there are losses in the visual or somatosensory systems, there is generally a poorer response to VRT. The goal and final level of recovery should be a return to most activities.
Conclusion
Patients with vestibular complaints commonly present to the otolaryngologist. A thorough evaluation an understanding of common vestibular disorders is important in their diagnosis and treatment. After diagnosis and treatment of acute symptoms, weaning of vestibular suppressants and specific pharmacotherapy should be instituted. Patients with persistent complaints or chronic, uncompensated disease will greatly benefit from vestibular rehabilitation instituted early during the course of disease.
Evaluation of Vestibular Function
Introduction
The otolaryngologist is frequently called upon to examine the dizzy patient in consultation when conservative measures have failed. Patients with the complaint of dizziness are among the most commonly seen by the primary care physician and the otolaryngologist. The role of the otolaryngologist in evaluating the dizzy patient is to determine the etiology of the dizziness through thorough evaluation and to assure appropriate treatment for the various underlying etiologies. The object of the evaluation is to separate possible causes into the categories of life threatening or benign, and to distinguish easily treatable causes from more complex causes.
This review will focus on specific clinical and vestibular laboratory tests that are currently available for evaluation of the dizzy patient. A complete knowledge of vestibular function is necessary for evaluation of the dizzy patient and for complete comprehension of this review. The reader is directed to previous grand rounds on vestibular function for reference.
The terms gain, phase, and symmetry are used to describe the various tests. Gain is the ratio of the amplitude of eye movement to the amplitude of head movement (stimulus). Phase is a parameter that describes the timing relationship between head movement and reflexive eye response. When the head and eyes are moving at exactly the same velocity in opposite directions, they are said to be exactly out of phase, or 180°. If the reflex eye movement leads the head movement, a phase lead is present, and if the compensatory eye movement trails the head movement, a phase lag is present. Symmetry is a comparison of the slow component of the nystagmus when rotated to the right compared with rotation to the left.
Screening Examinations of Vestibular Function
Although much has been written about the use of vestibular laboratory tests in the diagnosis of vestibular disorders, a complete history and physical including vestibular screening exams is the most effective tool in evaluating the dizzy patient. Studies have shown that the initial diagnosis by history and physical by experienced clinicians was confirmed by additional testing over 75% of the time. Screening tests are most effectively used to confirm the etiology of easily diagnosed conditions and to separate this group from those patients who need more extensive workup. A list of commonly preformed office exams are listed below. It is important that a complete review of systems be performed by the physician as conditions such as musculoskeletal instability can limit performing initial exams and may obligate laboratory testing.
Dix-Hallpike Maneuver – This test is the most well-known of office vestibular testing. It is used to provoke nystagmus and vertigo commonly associated with BPPV. In this test, the patient is seated on the exam table with head turned 45 degrees to the side and brought rapidly into the supine position with head hanging over the table. This maneuver allows maximal stimulation of the posterior SCC. Nystagmus that beats upwards, toward the stimulated ear with a rotary component to the affected ear, lasts 15-45 seconds, has a latency of 2-15 seconds, and fatigues are classic signs of BPPV. Nystagmus in the opposite direction can often be elicited by quickly sitting the patient up in the same position. Patients with the classic symptoms above can be treated with an Eply maneuver and do not need further vestibular testing.
Pneumatic Otoscopy – Positive and negative pressure applied to the middle ear with a pneumatic otoscope can elicit vertigo and nystagmus. This is known as Hennebert’s symptoms and signs. Hennebert’s sign can be seen in patients with perilymphatic fistula, syphilis, Meninere’s disease, and superior semicircular canal dehiscence syndrome.
Head Shake Nystagmus – This test is performed to evaluate asymmetry in the vestibular ocular reflex. The patient is instructed to tilt his or her head down 30 degrees to allow maximal stimulation of the horizontal canal and told to shake the head back and forth as quickly as possible for a period of 30 seconds. Immediately following cessation of movement, the eyes are opened and observed for nystagmus. Patients with unilateral vestibular dysfunction will have unopposed stimulation of the intact labyrinth which results in a slow-phase to the side of the lesion and a rapid nystagmus to the intact side. This response is usually brief. No nystagmus is expected in normal subjects. The presence of headshake nystagmus correlates well with peripheral vestibular function but has been identified in patients with cerebellar dysfunction. Patients with cerebellar dysfunction will often have a vertical component of the nystagmus. One study showed low sensitivity (27%) but good specificity (85%) in identifying vestibular dysfunction with this test.
Head Thrust Test – This test is used to evaluate for unilateral vestibular function. In this test, the patient’s head is turned 15-30 degrees from center and then rapidly rotated to the other side with the patient focusing on the examiner’s eyes. Patients with unilateral vestibular weakness will have a catch-up saccade when rotated rapidly to the side of the lesion. This test is rapidly and easily performed and can identify unilateral weakness of the vestibular system and as such is very useful clinically.
Dynamic Visual Acuity – This simple test can be used to sort out patients with bilateral vestibular weakness when chair testing is unavailable. With this test, a patient is asked to read the lowest line possible on a Snellen eye chart to establish a baseline visual acuity. This is followed by asking the patient to do the same task while rotating the head back and forth at a rate of 1-2 Hz. Loss of one line is considered normal, whereas loss of 2-3 lines suggests vestibular weakness. This test should be abnormal in patients with bilateral vestibular weakness and can be used to diagnose these challenging patients.
Eye movements – When testing cranial nerve function, the examiner should evaluate for smooth pursuit. Patients with erratic eye movements and saccades in various directions should be evaluated for CNS, specifically cerebellar dysfunction.
Romberg Testing – In this test, patients are asked to stand with feet together with arms folded around the chest or at the side. Patients are asked to stand like this with eyes open, and the amount of sway is compared to the same action with eyes closed. Patients with equal sway have proprioceptive or cerebellar lesions. More sway with eyes closed suggests vestibular lesions, most commonly with sway to the side of vestibular weakness. The test can be performed with foot next to toe to make the test more difficult and to bring out more subtle weakness.
Fukuda Stepping Test – In this test, the patient is asked to march in place with eyes closed and arms held out forward. In the original test, Fukuda had patients march 100 steps. Patients with vestibular weakness will rotate to the side of the weak labyrinth 45 degrees or more with 100 steps. Shorter versions of the test can be used to observe lesser amounts of rotation.
Orthostatic Hypotension – Many patients with the complaint of dizziness will have the diagnosis of orthostatic hypotension causing “light headedness.” Orthostatic hypotension is a reduction of systolic blood pressure of at least 20mm HG or diastolic blood pressure of at least 10mm HG within three minutes of standing. This can be found in asymptomatic individuals, but in patients with corresponding vertigo and drop in blood pressure, the diagnosis is reinforced. Symptoms of orthostatic hypotension include lightheadedness, blurred vision, weakness, fatigue, cognitive impairment, nausea, palpitations, tremulousness, headache, neck ache, and dizziness.
Dysdiadochokinesis Testing – Dysdiadochokinesis is the inability to make finely coordinated antagonistic movements. Patients with cerebellar lesions are often unable to perform these tests. The most common method is to have patients alternate slapping their knees with the palm and dorsum of the hand rapidly. Testing relies on the experience of the examiner who should be looking for patients with difficulty performing this maneuver above and beyond the normal variation. As such, it is only reliable when the physician has much experience in observing this test.
Tandem Gait Testing – Walking heel to toe in a circular pattern requires intact cerebellar function and as such is used by some examiners to screen for cerebellar dysfunction. The specificity of this examination is low because many causes other than cerebellar dysfunction may cause poor performance on tandem walking tests.
Voluntary Hyperventilation – Patients with suspected anxiety caused dizziness or patients with “hyperventilation syndrome” have been diagnosed in the past by having them voluntarily over breathe for a period of 90 seconds to 3 minutes to see if symptoms were reproduced. Recent tests have shown that the test has both poor sensitivity and specificity. Subjects results compared to normals have shown similar results. Some authors have suggested that patients with multiple sclerosis, acoustic neuroma, or vestibular neuritis can have symptoms provoked by hyperventilation, but these claims have not been validated by study.
Vestibular Laboratory Tests
After evaluation by history, physical, and the above mentioned screening tests, the clinician may still be unclear on the diagnosis and further testing may be warranted. Below is a review of the currently used vestibular tests with a detailed review of information obtained with each test. In addition, a brief review of the literature concerning indications and value of each test is provided.
Electronystagmography – ENG is the most widely used vestibular tests by otolaryngologist and relies on the vestibulo-ocular reflex to test the peripheral vestibular function and its ability to generate efficient voluntary eye movements necessary for maintaining visual contact with the environment. It consists of three subtests or subgroupings of tests: 1) oculomotor tests, 2) positional and positioning tests, 3) caloric tests.
The ENG begins with a calibration test in which the patient is asked to follow a dot on the wall, usually laser generated, that moves in a sinusoidal pattern to allow calibration of the eye movements with the monitor recording. In addition, gaze evoked and spontaneous nystagmus are evaluated. It is important to note that these eye movements can be more accurately evaluated by ENG due to the possibility of recording eye movements with closed eyes or with Frenzel glasses which eliminate visual fixation, and therefore suppress the nystagmus.
- Oculomotor tests – this first subdivision of ENG is evaluated by saccadic tracking, smooth pursuit tracking, and optokinetic tracking. The common thread of these tests is they all test eye movements that originate in the cerebellum. Abnormalities in these tests suggest a central neurological origin.
- Saccadic tracking – Saccades are rapid eye movements made to bring an object of interest into the center of the line of sight. Saccades are tested for accuracy, velocity, and latency. This test is performed with the patient concentrating on a randomly moving target. Latency, or the difference in time between the presentation of a new target and the initiation of eye movement, is considered normal if between 150-250ms in random patterns and shorter than 75ms when the pattern is predictable. Accuracy is measured by looking for eye movements that are equal in amplitude to the distance between the former object of interest and the new target. Hypometria is common in normal subjects and is the act of undershooting a target. If undershoot is less than 15% it is considered normal. Normal subjects with hypometria will quickly adjust to the target. Patients with normal cerebellar function will occasionally overshoot the target, called hypermetria. Hypometria and hypermetria are considered abnormal if they are consistently less then 75-80% or greater than 115-120% of the target value. Velocity is measured during saccade testing. There is no upper limit of normal as peak velocities have been measured as high as 700 degrees per second. Velocities slower than 400 degrees per second for large amplitude saccades and slower than 200 degrees per second for small amplitude saccades are considered abnormal.
- Smooth pursuit tests, also known as sinusoidal tracking, test the ability of the patient to accurately and smoothly pursue a target. In these tests, a target is rotated back and forth in a sinusoidal pattern. Gain is measured and the eye movements are compared to the movement of the target. It is important to note that saccade movements are eliminated from the calculations of gain. Asymmetrical eye movements are highly suggestive of CNS disease.
- Optokinetic tracking – when a patient is spinning, he relies on the stimulation from the vestibular system and optokinetic nystagmus to allow steady focus on objects as they move in a circular pattern around him. As the patient’s vestibular system fatigues with stimulation, the optokinetic system is solely responsible for the stabilization of the visual field. ENG tests the optokinetic tracking of targets by passing a light rapidly in front of a patient from one direction to the other and asking the patient to count the lights. This is done first in one direction, then the other. Asymmetries are noted and are signs of central nervous system dysfunction. Various tests have been done that have shown unacceptable false positive results with this test. Patients with abnormalities in the optokinetic portion of the ENG may not need further neurological work-up.
Positional and positioning tests – Unlike the previous subset of tests, this portion of the ENG tests for abnormalities in the vestibular system. Positional tests are performed to determine whether the vestibular system responds normally and symmetrically to changes in head position. This is based on the concept that when a person receives an insult to one labyrinth, compensation occurs by constant stimulation. It is natural that the subject will become compensated in the position that he most frequently uses, namely the upright position. When placed in different positions, dizziness and nystagmus may occur as a result of incomplete compensation in that particular position. Patients with dizziness and no nystagmus provoked by different positions must have non-vestibular etiologies considered. Also, vertical nystagmus or multidirectional nystagmus is highly suggestive of central disorders. One study noted that ¼ of patients with atypical positional nystagmus were found to have CNS disorders or vascular insufficiency. Conversely, ¾ of patients with these findings have idiopathic or benign causes. When performing positional tests, it is important to eliminate the influence of neck flexion on results as flexion can cause vascular insufficiency that can provoke symptoms suggestive of peripheral vestibular disorders. Positional testing is used to determine the presence of BPPV. It is the movement that generates the response and not the position. BPPV is most commonly the result of otoliths in the posterior canal. Stimulation using the Dix-Hallpike maneuver commonly causes torsional nystagmus which is difficult to measure if traditional ENG is used. As such, several video systems have been developed to record eye movement to be reviewed by the examiner later. It is important to note that patients may fatigue the response, and patients with true BPPV may test negative on the initial visit. It is therefore important in both ENG testing and clinical testing that patients with a strong history of BPPV be tested on multiple occasions using the Dix-Hallpike maneuver.
Caloric Testing – This is the only vestibular test that stimulates one side of the vestibular system at a time. In this test, patients are placed in a 30 degree from prone position to allow the horizontal semicircular canal to be in a vertical orientation. Warm and cold water or air is then flushed into the external auditory canal at 7 degrees above or below body temperature. Varying temperature causes a non-physiologic stimulation of one labyrinth that may evoke vertigo, nystagmus, and occasionally nausea and vomiting. Warm water for example causes the perilymph to rotate towards the ampula, resulting in stimulation of the ipsilateral labyrinth and a drift of the eyes away from the stimulated side. The eyes compensate with a saccade toward the stimulated side. The opposite occurs with cold water stimulation. The mnemonic COWS is used to remember the direction of nystagmus (Cold Opposite Warm Same). Ice water irrigations can be used if milder stimulation does not result in nystagmus. Because caloric irrigation results in stimulation analogous to head movements of 0.002 to 0.004 Hz, significant vestibular asymmetry can exist in the presence of normal caloric responses as real-life head movements are in the 1 to 6 Hz range. Visual fixation should reduce the strength of caloric responses in the presence of normal CNS function. Failure to suppress nystagmus 50-70% with visual fixation is abnormal.
Rotational Chair Testing
A Rotational Chair Test is performed by securing the patient to a rotary chair which rotates at various speeds and directions while eye movements are recorded. Sinusoidal harmonic acceleration tests at several frequencies, visual-vestibular interaction tests, VOR suppression tests, and step velocity tests are frequently performed using the rotary chair test.
- Sinusoidal Harmonic Acceleration – In this test, the chair is rotated at frequencies as low as 0.01 Hz and as high as 1.28 Hz. These frequencies are tested and then doubled in consecutive fashion to achieve harmonic acceleration. The chair is moved in either direction to stimulate both labyrinths equally. Gain, phase, and symmetry are measured. Gain at lower frequencies is augmented by the visual pursuit system and does not equal head movement. As higher frequencies are approached, gain and head movement are nearly equal. Patient’s results are compared to the manufactures’ norms. Patients with two consecutive frequencies out of range are considered abnormal. Patients with unilateral vestibular lesions will often have spontaneous nystagmus in darkness which can create a bias and asymmetry towards the side of the lesion, but this is not always true. After compensation has occurred, gain tends to normalize, but phase generally remains asymmetric. This is the most common abnormality in sinusoidal harmonic acceleration testing. More useful are the results obtained from this test in bilateral vestibular hypofunction. Patients with bilateral vestibular lesions will often demonstrate reduced gain across all frequencies. This may be the first abnormality in patients with vestibular loss associated with gentamicin use. Cerebellar dysfunction is suggested by situations in which VOR gain exceeds the velocity of head movements.
- Visual-Vestibular Interaction Test – In this test the patient is rotated in both directions while being asked to track objects on the wall. The goal for the patient is to match eye movement speeds with the speed of the chair. This tests the patient’s ability to compensate for deficits in the vestibular ocular reflex with voluntary pursuit. Compensatory eye tracking strategies have utility in aiding recovery from vestibular deficits. This test identifies the patient’s ability to use tracking for rehabilitation.
- Vestibular Ocular Reflex Suppression Test – In this test, the patient’s ability to use visual fixation to suppress the VOR is tested. Patients are asked to fixate on an object spinning at the same speed as the chair, often a thumb held in front of the face. Patients should be able to suppress 90% of VOR gain. Failure to do so suggests a cerebellar lesion.
- Step Velocity Testing – In this test the chair is accelerated in one direction at a rate of 100 degrees/ seconds squared to a constant velocity. When sustained rotation is reached, the intensity of the nystagmus response will gradually decrease. The slow component of velocity is recorded as is the time constant. The time constant is the point in seconds at which the slow component velocity decreases to 37% of its peak after acceleration is stopped. The time constant is a measure of the velocity storage mechanism which is the continuation of neural response and persists after the fluid dynamics of the labyrinth have been exhausted. Decreased time constant and reduced gain on step velocity testing are sensitive but nonlocalizing findings for vestibular dysfunction.
Posturography
The term posturography may be used to describe any test of postural stability or standing balance, but is most often used to describe computerized dynamic posturography. Where as ENG and rotational chair testing are designed to evaluate the horizontal VOR by stimulating the horizontal semicircular canals, posturography evaluates other components of balance. Balance requires cerebellar integration of the information from vestibular, visual and somatosensory organs. Dysfunction of any of the necessary components of balance results in stronger reliance on other peripheral sensors for maintenance of balance. Posturography systematically takes away one or more sensory components to evaluate which component the patient is reliant upon for balance. This is accomplished through one of six conditions:
- Condition one – Stable platform with eyes open in a stable visual environment (patient has full use of all information: visual, vestibular, and somatosensory)
- Condition two – Stable platform with eye closed (patient must rely on vestibular and somatosensory information)
- Condition three – Stable platform with moving visual surroundings (patient must suppress a false sense of visually induced movement and rely on vestibular and somatosensory inputs)
- Condition four – Unstable platform with eyes open in a stable visual environment (patient must rely on vestibular and visual inputs)
- Condition five – Unstable platform with eyes closed (patient must rely on vestibular input only because visual and somatosensory feedback have been eliminated)
- Condition six – Unstable platform and unstable visual environment (patient must rely on vestibular input alone and suppress a false sense of visually induced movement.
Certain patterns of dysfunction are associated with specific deficits. Poor performance in conditions five and six are seen in patients with vestibular dysfunction. A visual preference is seen when patients positively test for conditions three and six. Because patients in this visual preference group are not unstable when vision is taken away (condition 5), vestibular dysfunction is not present. These patients have visual vestibular integration problems and need neurological referral. Patients with poor balance in conditions four, five, and six have a somatosensory dependence pattern. They should be referred for physical therapy assessment of strength and neurologic assessment of sensation of the lower extremities. When patients fall in conditions two, three, five, and six, a visual dependence pattern suggests that patients are overly reliant on visual information. Therapy for these patients incorporates gradual reduction in visual feedback while performing exercises. Malingering patients often show difficulty in all conditions equally. This is different from the expected poorer results in more difficult situations.
It has been suggested by Allum that five different patients benefit from posturography:
- Patients with chronic disequilibrium and normal clinical examinations.
- Patients who may be malingering.
- Patients with cervicogenic disequilibrium.
- Patients with suspected multifactorial disequilibrium.
- Patients with poorly compensated vestibular injuries.
Conclusion
The dizzy patient is most effectively evaluated by complete history, physical and clinical examination by a physician experienced in vestibular and neurological disorders. For the small subset of patients that need further testing for accurate diagnosis of their underlying disorder, several tests are available to the clinician for evaluation. Further study is needed to determine the efficacy of these tests and their proper use.
PHYSIOLOGY OF THE VESTIBULAR SYSTEM
Within the inner ear are specialized sensory receptors responsible for the perception of the forces associated with head movement and gravity. Control centers within the brainstem integrate this information along with other biologic signals derived from vision and proprioceptive sensors in the final determination of an individual’s orientation in three-dimensional space. Although anatomically developed and responsive at birth, the vestibular system matures along with other senses in the first 7 to 10 years of life. Recognition of the head’s movement relative to the body is provided by the linear (otolithic macula) and angular (semicircular canals) acceleration receptors of the inner ear. Electrical activity generated within the inner ear travels along the vestibular nerve (primary afferent neuronal pathway) to the central vestibular nuclei of the brainstem, forming second-order neuronal pathways that become the vestibulo-ocular reflex (VOR), the vestibulospinal tracts, and the vestibulocerebellar tracts. Pathways derived from vestibular information also travel to the brainstem emetic centers, which serves to explain vegetative symptoms such as nausea, vomiting, and perspiration that a patient typically experiences following an acute unilateral vestibular loss.
Disruption of peripheral (inner ear and the vestibular nerve) or central vestibular pathways as a result of, for example , trauma, ototoxicity, or surgical deafferentation leads to the patient experiencing a distortion in orientation. The patient often uses the term“dizziness,” which is an all-encompassing yet relatively nonspecific term that can include symptoms such as giddiness, lightheadedness, and floating sensation. Clinically, the term “vertigo” is best suited to describe a precise type of dizziness—a hallucination of movement involving oneself (subjective vertigo) or the surrounding environment (objective vertigo) that is apt to occur when there is an acute interruption of vestibular pathways.
Of all the human vestibular pathways, the VOR remains the most important and most studied. At its simplest level the VOR is required to maintain a stable retinal image with active head movement. When an active head movement is not accompanied by an equal but opposite conjugate movement of the eyes, retinal slip occurs. When the VOR is affected bilaterally (as could occur from systemic aminoglycoside poisoning) patients characteristically complain of visual blurring with head movement, better known as oscillopsia, in addition to having significant complaints of imbalance and ataxia.3 True vertigo is typically not a feature of a bilateral peripheral vestibular loss.
Anatomy of the Vestibular System Peripheral Vestibular System
The peripheral vestibular system includes the paired vestibular sensory end-organs of the semicircular canals (SCCs) and the otolithic organs. These receptors are found within the fluid-filled bony channels of the otic capsule (the dense endochondral-derived bone that surrounds the labyrinth and cochlea) and are responsible for perception of both the sense of position and motion. The vestibular nerve (both superior and inferior divisions of the VIIIth nerve) is the afferent connection to the brainstem nuclei for the peripheral vestibular system.
Perception of angular accelerations is chiefly the responsibility of the three paired SCCs (superior, posterior, and lateral). Within the ampullated portion of the membranous labyrinth are the end-organs of the cristae, containing specialized hair cells that transducer mechanical shearing forces into neural impulses.
Histologically the hair cells of the ampulla are located on its surface. Their cilia extend into a gelatinous matrix better known as the cupula, which acts like a hinged gate between the vestibule and the canal itself. The otolithic organs of the utricle and the saccule are found within the vestibule. Chiefly responsible for the perception of linear accelerations (eg, gravity, deceleration in a car), their end-organs consist of a flattened, hair cell–rich macular area whose cilia project into a similar gelatinous matrix. The matrix, however, differs from the matrix associated with the SCCs in its support of a blanket of calcium carbonate crystals better known as otoliths, which have a mean thickness of approximately 50 μm.
Information from the vestibular end-organs is transmitted along the superior (which receives information from the superior, horizontal SCCs and utricle) and inferior (which receives information from the posterior SCC and saccule) divisions of the vestibular nerve. Although its role is primarily afferent in the transmission of electrical activity to the central vestibular nuclei of the brainstem, an efferent system does exist that probably serves to modify end-organ activity. Each vestibular nerve consists of approximately 25,000 bipolar neurons whose cell bodies are located in a structure known as Scarpa’s ganglion, which is typically found within the internal auditory canal (IAC). Type I neurons of the vestibular nerve derive information from corresponding type 1 hair cells, whereas type II neurons derive information from corresponding type 2 hair cells at its simplest.
Central Vestibular System
Primary vestibular afferents enter the brainstem dividing into ascending and descending branches. Within the brainstem there appears to exist a nuclear region with four distinct anatomic types of second-order neurons that have been traditionally considered to constitute the vestibular nuclei. It appears, however, that not all these neurons receive input from the peripheral vestibular system.4,7 The main nuclei are generally recognized as the superior (Bechterew’s nucleus), lateral (Deiters’ nucleus), medial (Schwalbe’s nucleus ), and descending (spinal vestibular nucleus).
Functionally, in primate models, the superior vestibular nucleus appears to be a major relay station for conjugate ocular reflexes mediated by the SCCs. The lateral vestibular nucleus appears to be important for control of ipsilateral vestibulospinal (the so-called “righting”) reflexes. The medial vestibular nucleus, because of its other connections with the medial longitudinal fasciculus, appears to be responsible for coordinating eye, head, and neck movements. The descending vestibular nucleus appears to have an integrative function with respect to signals from both vestibular nuclei, the cerebellum, and an amorphous area in the reticular formation postulated to be a region of neural integration. Commonly referred to as the “neural integrator” among neurophysiologists, it is responsible for the ultimate velocity and position command for the final common pathway for conjugate versional eye movements and position.
The vestibular nerve in part also projects directly to the phylogenetically oldest parts of the cerebellum—namely, the flocculus, nodulus, ventral uvula, and the ventral paraflocculus—on its way directly through the vestibular nucleus. Better known as the vestibulocerebellum, this area also receives input from other neuronal pathways in the central nervous system (CNS) responsible for conjugate eye movements, especially smooth-pursuit eye movements, which, in addition to the VOR, are responsible for holding the image of a moving target within a certain velocity range on the fovea of the retina. The Purkinje’s cells of the flocculus are the main recipients of this information, of which some appears to be directed back toward the ipsilateral vestibular nucleus for the purposes of modulating eye movements in relation to gaze (eye in space) velocity with the head still or during combined eye–head (vestibular signal-derived) tracking. Important for cancelling the effects of the VOR on eye movement when it is not in the best interest of the individual (think of twirling ballet dancers or figure skaters and how they can spin without getting dizzy), the vestibulocerebellum is also important in the compensation process for a unilateral vestibular loss.
The Hair Cells
The fundamental unit for vestibular activity on a microscopic basis inside the inner ear consists of broadly classified type 1 and 2 hair cells. Type 1 hair cells are flask-shaped and surrounded by the afferent nerve terminal at its base in a chalicelike fashion. One unique characteristic of the afferent nerve fibers that envelop type 1 hair cells is that they are among the largest in the nervous system (up to 20 μm in diameter). The high amount of both tonic (spontaneous) and dynamic (kinetic) electrical activity at any time arising from type 1 hair cells has probably necessitated this feature for the neurons that transfer this information to the CNS. Type 2 hair cells are more cylindrical and at their base are typically surrounded by multiple nerve terminals in contradistinction.
Each hair cell contains on its top a bundle of 50 to 100 stereocilia and one long kinocilium that project into the gelatinous matrix of the cupula or macula. It is thought that the location of the kinocilium relative to the stereocilia gives each hair cell an intrinsic polarity that can be influenced by angular or linear accelerations. It is important to realize that an individual is born with a maximum number of type 1 and 2 hair cells that cannot be replaced or regenerated if lost as a result of the effects of pathology (eg, ototoxicity or surgical trauma) or aging (the postulated presbyvestibular dropout from cellular apoptosis). Presumably the same process holds for the type I and II neurons that comprise the vestibular nerve.
Applied Physiology
At the microscopic level, movements of the head or changes in linear accelerations deflect the cupula or shift the gelatinous matrix of the otolithic organs with its load of otolithic crystals that will either stimulate (depolarize) or inhibit (hyperpolarize) electrical activity from type 1 and 2 hair cells. Displacement of the stereocilia either toward or away from the kinocilium influences calcium influx mechanisms at the apex of the cell that causes either the release or reduction of neurotransmitters from the cell to the surrounding afferent neurons. The electrical activity generated is then transferred along the vestibular nerve to the vestibular nuclei in the brainstem. Information above the tonic (spontaneous) firing rate of the type 1 hair cells transmitted along type I neurons is largely thought to have a stimulatory effect in contrast to a more inhibitory effect attributable to type 2 hair cells and type II neurons.
The SCCs largely appear to be responsible for the equal but opposite corresponding eye-to-head movements better known as the VOR. The otolithic organs are primarily responsible for ocular counter-rolling with tilts of the head and for vestibulospinal reflexes that help in the maintenance of body posture and muscle tone.
In order to ultimately produce conjugate versional VOR-mediated movements of the eyes, each vestibular nucleus receives electrical information from both sides that is exchanged via the vestibular commissure in the brainstem. The organization is generally believed to be specific across the commissure. Neurons in the right vestibular nucleus, for example, that receive type I input from the right horizontal SCC project across the commissure to the neurons found in the left vestibular nucleus that are driven by the left horizontal SCC receiving contralateral type II input and vice versa.
Key Concepts
According to Leigh and Zee’s seminal text, the key concepts of vestibular physiology can be best appreciated in the context that “the push–pull pairings of the canals, the resting vestibular tone and exchange of neural input through the vestibular commissure maximize vestibular sensitivity in health and provide a substrate for compensation and adaptation.”
VOR Gain
In order to maintain a stable retinal image during head movement, the eyes should move in an equal but opposite direction to head movement. Anything less than unity (corresponding eye movement/head movement) may result in the perception of visual blurring with head movement—oscillopsia being the classic symptomatic complaint of an individual with a bilateral peripheral vestibular loss as might result from gentamicin vestibulotoxicity.
Nystagmus
Defined as a rhythmic to-and-fro, back-and-forth movement of the eyes, nystagmus represents the cardinal sign of unilateral peripheral vestibular or central vestibular dysfunction.
In an acute unilateral loss of peripheral vestibular activity that might occur from topical aminoglycoside drops or certain disinfectant surgical preparation solutions used in the presence of a tympanic membrane defect, injury to the end-organ causes a difference in neural activity between the left and right vestibular nuclei. Should the push–pull pairings of the canals be affected as a result of pathology, the eyes are typically driven with a slow movement toward the affected side only to be corrected by a fast corrective saccade generated within the CNS away from the side of the lesion in a repetitive fashion. Although somewhat misguided, the direction of the nystagmus by convention refers to the fast phase, typically away from the side of the lesion under circumstances of an acute unilateral peripheral vestibular loss.
Habituation and Adaptation
In humans the CNS may habituate (show a reduced response) the VOR depending on the environmental circumstances. This may happen in individuals who are blind or in those exposed to constant velocity rotations or continuous low-frequency oscillations (such as on a ship). The mechanisms for adaptation or the adaptive plasticity of the VOR are usually visually driven and have been experimentally studied by subjects wearing reversing prisms. This phenomenon is frequently experienced by those wearing new prescriptive glasses with the explanation that “they take some time to get used to.” Eventually one adapts to the new lenses as the gain of the VOR changes accordingly. The same holds true to some extent for those with a unilateral peripheral vestibular loss, where the gain can be somewhat influenced, though not perfectly.
Compensation
Clinical improvement following acute unilateral peripheral vestibular deafferentation requires the presence of intact central vestibular connections primarily at the level of the vestibulocerebellum.4,7 The loss of tonic or spontaneous vestibular activity from the endorgan is ultimately replaced by the development of spontaneous electrical activity arising within the vestibular nuclei of the affected side.14 At rest the asymmetries that would be expected from the push–pull effects from the canals are kept in check, and as a result there is the gradual resolution of the once-present spontaneous nystagmus. Quick head movements producing changes in the dynamic electrical activity, however, can never be completely compensated through this mechanism on the affected side, and a bilateral loss of inner ear function never does despite the insertion of midrotation corrective saccades. For a more detailed explanation of the phenomenon of compensation and why it often fails in the setting of a bilateral vestibular loss,“Monitoring Vestibular Toxicity.”
Clinical Manifestations of Vestibular Dysfunction
Loss of vestibular function is associated with several signs and symptoms.
Unilateral Peripheral Vestibular Loss
With a loss of unilateral vestibular function the patient acutely experiences the sensation of true vertigo from interruptions of VOR pathways and tends to lie perfectly still, as any movement aggravates vegetative symptoms such as nausea and vomiting that arise from the emetic centers. Nystagmus beating away from the side of lesion is the cardinal physical sign that obeys Alexander’s law (the quick phase of the nystagmus induced by the imbalance in activity at the level of the vestibular nuclei is greatest in amplitude and frequency when the eyes are turned away from the side of the lesion). Interruption in vestibulospinal tract pathways causes the patient to fall or list toward the affected side. Findings of ipsilateral hemispheric cerebellar dysfunction presenting with behaviors such as past-pointing, an inability to perform rapid alternating movements (dysdiadochokinesis), and gait ataxia reflect acute vestibulocerebellar tract involvement. Features distinguishing peripheral from central mediated nystagmus can be found.
With compensation (implying the existence of a normal functioning CNS and contralateral peripheral vestibular system) there may be minimal symptomatology that is only brought out by very rapid head movements. The spontaneous nystagmus disappears, vegetative symptoms resolve, gait improves, and in the case of a chronic condition the patient may experience only a slight imbalance when turning quickly. Bilateral Peripheral Vestibular Loss Vertigo is not a feature of a bilateral vestibular loss even when it occurs in an acute fashion. Injury to the endorgans as might occur in systemic aminoglycoside vestibulotoxicity causes a bilateral loss of function that tends to be electrically symmetric at the level of the vestibular nuclei in the brainstem. Instead the patient tends to complain of oscillopsia and imbalance. The gait is typically broad-based and ataxic, especially with eyes closed. Falls are not infrequent and in many instances the patient requires assistive devices for ambulation or is relegated to a wheelchair. Compensation is generally unlikely to occur despite the best efforts of vestibular rehabilitation therapy and a greater reliance on information from visual and proprioceptive receptors.
Special Clinical Tests of Vestibular Function
The following clinical tests are used at the bedside in the assessment of vestibular function and are specific for the VOR. A summary can be found. High-Frequency Head Thrust or Halmagyi Maneuver Perhaps the most specific test for horizontal VOR function, the high-frequency head thrust, shares the same physiologic basis as the “doll’s eye” maneuver in neurology. During a quick head movement to the normal side with the patient focusing on a target, there should be almost perfect compensatory conjugate versional movement of the eyes in an equal but opposite direction to head movement. When a defect occurs in the VOR, quick movements of the head are usually associated with incomplete compensatory conjugate eye movements often requiring refixation saccades to stabilize gaze. Not surprisingly patients with a unilateral vestibular loss often volunteer subjective blurring of vision when they move their head to the affected side.
Although a positive head thrust maneuver is always indicative of pathology somewhere along the course of VOR, false-negative findings can arise. This can occur when an individual throws in corrective midrotation saccades that are imperceptible to the human eye. Identification with advanced eye-movement recording systems such as magnetic scleral coil search studies would typically be required.
Head Shake Test
Rapid horizontal head shaking for 15 to 20 seconds occasionally results in horizontal post-headshake nystagmus usually (but not always) directed away from the side of a unilateral vestibular loss.19 Frenzel’s glasses are generally worn to prevent ocular fixation and suppression of nystagmus by vision.Headshake nystagmus is generally thought to occur when asymmetries in resting vestibular tone are exaggerated at the level of the postulated central velocity storage mechanism in the brainstem.
Dynamic Visual Acuity (Oscillopsia Testing)
Complaints of oscillopsia are best assessed by asking the patient to read the lowest line possible on a standard Snellen’s chart. While repetitively shaking the head in the horizontal plane (at a frequency > 2 Hz) the patient is asked to read the lowest line possible for comparison purposes. A loss of more than five lines during active head-shaking is considered definitely clinically significant for a bilateral vestibular loss. The test can also be performed using an optotype such as the letter “E” (the so-called dynamic illegible “E,” or DIE, test) presented in random orientations and different sizes on a chart or monitor. Comparison between the static and dynamic optotypes correctly identified has been used to determine if a bilateral vestibular loss exists.
VOR Suppression Testing (Fixation of Vestibular-Induced Nystagmus)
This test assesses the ability of the CNS to suppress a vestibular signal. It can be assessed by asking patients to follow with the head in the same direction an object that rotates (eg, patients look at their outstretched hands held together while seated in a chair that rotates). If the vestibulocerebellum is intact then the eyes should remain stable in the orbit from visual fixation and suppression of the VOR. In central vestibular pathology, oculomotor testing typically reveals pursuit eye movements that are saccadic associated with the presence of a breakthrough nystagmus during head rotations as fixation is incomplete.24 VOR suppression testing is somewhat analogous to the parallel phenomenon of failure of fixation suppression during caloric testing when visual fixation does not suppress caloric-induced nystagmus.