RHINOLOGY
CONGENITAL MIDLINE NASAL MASSES
Introduction
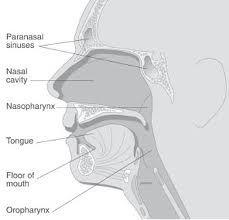
Congenital midline nasal masses include nasal dermoids, nasal gliomas, and encephaloceles. These are rare congenital anomalies, estimated to occur in 1:20,000 to 40,000 births. Although rare, these disorders are clinically important because of their potential for connection to the central nervous system. Biopsy of a lesion with an intracranial connection can lead to meningitis or cerebrospinal fluid leak. The treatment of these masses is surgical excision. Preoperative knowledge of an intracranial connection allows for neurosurgical consultation and planning for craniotomy. The differential of a midline nasal mass includes inflammatory lesions, traumatic deformity, benign neoplasms, malignant neoplasms, and congenital masses. Dermoid sinus cysts present as a mass on the dorsum of the nose or intranasally, with a pit or sinus tract opening on the nasal dorsum, hair around the external opening, and discharge of pus or sebaceous material. Nasal gliomas are firm masses which are nonpulsatile, present on the nasal dorsum and/or arise from the lateral nasal wall, have telangiectasias of the overlying skin, and do not enlarge with bilateral compression of the internal jugular veins (Furstenberg test). Encephaloceles may present as nasal broadening and/or as a blue, pulsatile, compressible mass near the nasal bridge which transilluminates, enlarges with crying or with bilateral compression of the internal jugular veins, or as an intranasal mass arising from the cribriform plate. To understand the development of congenital midline nasal masses, knowledge of the normal embryological development of the nose is important.
Embryology
The most critical period in the embryology of the face is during the first twelve weeks of fetal development. Between the third and fourth weeks of fetal growth the neural fold develops and forms the neural tube on the dorsal aspect of the embryo. Closure of the neural groove begins in the middle of the embryo and extends in a cranial and caudal direction. A key element in understanding development of the face, including the nose, is the importance of the neural crest cells. As the neural tube is forming by closure of the neural groove, neural crest cells migrate laterally and anteriorly around the eye to the frontonasal process. In most of the body the neural crest cells are involved in forming ectodermal components. However, in the neural crest cells in the face primarily form mesenchymal cells which provide the bone, cartilage, and muscles of the face.
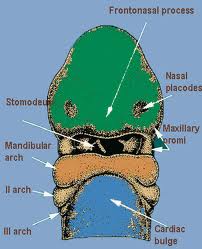
The nose is formed from the frontonasal process and two nasal placodes which develop dorsal to the stomadeum (primitive mouth). The nasal placodes become more prominent and consist of a medial and lateral process. The medial processes approach one another and eventually fuse in the midline. The lateral processes become less prominent as the maxillary process fuses with them. A deep groove in this region, called the nasal-maxillary groove becomes the nasolacrimal duct. As the external nose is developing, other neural crest cells migrate through the frontonasal process to form the posterior septum, ethmoid bone, and sphenoid. The nasal septum develops around week five from the frontonasal process, growing in an anterior-posterior direction.
During formation of the skull base and nose the mesenchymal structures are formed from several centers which will eventually fuse and begin to ossify. Before their fusion there are recognized spaces between these elements which are important in the development of congenital midline nasal masses. These include the fonticulus frontalis, the prenasal space, and the foramen cecum. The fonticulus nasofrontalis is the space between the frontal and nasal bones. The prenasal space is between the nasal bones and the nasal capsule (the precursor of the septum and nasal cartilages). During fetal development these spaces are normally closed by fusion and ossification. Abnormal development of these structures is thought to be involved in the formation of dermoids, gliomas, and encephaloceles of the nose. This will be discussed with each particular anomaly later in this paper.
Dermoid Sinus Cysts
Nasal dermoid sinus cysts are the most common of the congenital midline nasal masses. Many present at birth but some are not found until later in childhood or even adulthood when they become symptomatic. They can occur as an isolated cyst or with a sinus tract opening to the skin. They constitute 1-3% of all dermoids but 3-12% of dermoids of the head and neck. In one study dermoid and epidermoid cysts together accounted for 61% of nasal masses in a review of 109 cases. Dermoids are related to epidermoid cysts but contain both ectodermal and mesodermal elements (adnexal structures such as hair follicles and sebaceous glands).
Dermoid sinus cysts of the nose present as a midline nasal pit, fistula, or infected mass located anywhere from the glabella to the nasal columella. Usually, nasal dermoids terminate in a single subcutaneous tract which can sometimes have hair at the opening. They may secrete sebaceous material or pus, become intermittently inflamed, form an abscess, cause osteomyelitis, broaden the nasal root or bridge, lead to meningitis, or form a cerebral abscess. Connection with the central nervous system has been variably reported to occur from 4-45%. Suspicion of intracranial involvement should remain high. Associated congenital anomalies occur in 5-41% of cases which include aural atresia, mental retardation, spinal column abnormalities, hydrocephalus, hypertelorism, hemifacial microsomia, albinism, corpus callosum agenesis, cerebral atrophy, lumbar lipoma, dermal cyst of the frontal lobe, coronary artery anomaly, cleft lip and palate, tracheoesophageal fistula, cardiac, genital, and cerebral anomalies. There is no known syndromic association of these anomalies.
A widely accepted theory of dermoid sinus cyst development is the prenasal space theory. According to this theory, during normal development a projection of dura protrudes through the fonticulus frontalis or inferiorly into the prenasal space. This projection normally regresses but if it does not the dura can remain attached to the epidermis and result in trapped ectodermal elements.
Gliomas
Gliomas are made of neuroglial elements consisting of glial cells in a connective tissue matrix with or without a fibrous connection to the dura. There is no fluid filled space connected to the subarachnoid space. These lesions usually present as a red or bluish lump at or along the nasomaxillary suture, or as an intranasal mass. They are characteristically firm, noncompressible, do not increase in size with crying, and do not transilluminate. The overlying skin may have telangiectasias. They can be associated with a widened nose or with hypertelorism secondary to growth of the mass.
Intranasal gliomas most often arise from the lateral wall of the nose or less often from the nasal septum. Sixty percent are extranasal, 30% intranasal, and 10% are both. Overall, 15% are connected to the dura. The intranasal type is more often associated with dural attachment (35%) than the extranasal type (9%). They are more common in males by a 3:1 ratio although the significance of this has not been established.
The embryological development of nasal gliomas is similar to nasal dermoids. Abnormal closure of the fonticulus frontalis can lead to an ectopic rest of glial tissue being left extracranially. This is similar to the mechanism for the formation of encephaloceles, however there is not always an intracranial connection to a glioma and there is by definition an intracranial connection to an encephalocele. This will be further discussed in the following section.
Encephaloceles
Encephaloceles are extracranial herniations of the meninges and/or brain which maintain a subarachnoid connection. If it contains only meninges it is termed a meningocele, when it also contains brain tissue it is called a meningoencephalocele. Ingraham and Matson (11) divided encephaloceles into three categories: occipital, sincipital, and basal. Occipital are the most common at 75%. Sincipital are frontonasal lesions which present as a mass over the nose, glabella, or forehead. The intracranial connection is usually anterior to the cribriform plate. Suwanwela and Suwanwela (21) divided nasal encephaloceles into nasofrontal, nasoethomoidal, and naso-orbital lesions based on the projection of the mass between the nasal and frontal bones, along the side of the nose, or into the medial orbit. He reported that nasofrontal encephaloceles occur directly anterior with short necks and could possibly be excised via an external approach while nasoethmoidal and nasoorbital have long necks and necessitate intracranial closure. Basal lesions make up about 10% of lesions and present as an intranasal or nasopharyngeal mass. Basal lesions herniate either through the cribriform plate or posterior to it which explains their presentation in the nose instead of externally. They rare at 1:35,000 live births in Western Europe, America, Australia, Japan, China, and India but more common at 1:6000 live births in Southeast Asia and Russia. Encephaloceles are often bluish, soft, compressible masses which can be transilluminated. They enlarge with crying or the Valsalva maneuver. A characteristic sign is the Furstenberg test, which is enlargement with compression of the internal jugular veins. They also can cause a widening of the nose or hypertelorism. Intranasal encephaloceles originate medially in the nasal cavity as opposed to gliomas which most often originate laterally.
The embryologic development of encephaloceles is the same as that for gliomas. Failure of the fonticulus frontalis to close properly can lead to a herniation of intracranial contents which maintains its connection to the subarchnoid space. This connection with the central nervous system and the possibility of containing brain tissue make encephalocele and important entity to rule out when a midline nasal mass is found.
Evaluation
The evaluation and management of congenital midline nasal masses starts with a complete history and physical exam. Many of these lesions present early in life but adults may also be found who are undiagnosed with these lesions. Dermoids often can present with repeated infection or drainage, a visible sinus tract, are more solid, noncompressible, and do not transilluminate. Nasal gliomas are also firm, noncompressible, and do not transilluminate but may have overlying telangiectasia. Encephaloceles may be bluish or red, soft, compressible, enlarge with crying, and have a positive Furstenberg test. With intranasal lesions, gliomas arise from the lateral wall while encephaloceles arise more medially. According to Haafiz, an intranasal probe can often be passed medial to a glioma but not to an encephalocele(9). The distinction between glioma and encephalocele is important because while 15% of gliomas have an intracranial connection, all encephaloceles have an intracranial connection.
When a dermoid, glioma, or encephalocele is a suspected diagnosis a biopsy should not be performed before an intracranial connection is ruled out because of the risk of causing meningitis or CSF leak. The majority of these lesions are found in children, and a high index of suspicion is required, especially for a unilateral intranasal mass. The diagnosis is confirmed by CT and/or MRI imaging. Image findings include soft tissue mass, fluid filled cyst, intracranial mass, enlargement of the foramen cecum, and distortion of the crista galli. CT imaging better delineates bony abnormalities while MRI is valuable to identify an intracranial connection. The findings on CT consistent with intracranial involvement are an enlarged foramen cecum or bifidity of the crista galli. Although these findings are consistent with intracranial involvement they are not diagnostic. According to Pensler et al., these findings are only conclusive if they are absent, eliminating an upward intracranial connection. MRI provides better soft tissue detail and ability to visualize in the sagittal plane. Denoyelle reviewed thirty-six children with nasal dermoid sinus cysts and recommends an MRI scan to confirm any suspected intracranial extension following a CT scan. In his series two patients had false positive CT scan evidence of intracranial connection which was not found at surgery(6). Bloom et al. reviewed ten patients with nasal dermoids, reporting CT results falsely positive for intracranial extension in one of six cases and indeterminate in one of six cases. Due to the increase cost of two tests, delay in diagnosis, and added risk of additional anesthesia for additional imaging, they recommend MRI as the initial imaging study. Schlosser et al., reported three cases of the use of preoperative three dimensional CT scanning. They found it useful in a case of an encephalocele with a large anterior cranial floor defect to demonstrate the full extent of the defect and to provide images to use in counseling of the parents more easily understood than conventional two dimensional CT (19).
Surgical Treatment
The treatment of nasal dermoids, gliomas, and encephaloceles is by complete surgical excision. Early surgical intervention is recommended to avoid further distortion of the nose or bony atrophy caused by growth of the mass or recurrent inflammation. Other complications are abscess formation, osteomyelitis, and meningitis with those lesions with an intracranial connection. The entire lesion along with any fistulous tract must be excised in order to prevent recurrence. Denoyelle et al. reported a recurrence rate of 5.5% (two of thirty-six patients) for nasal dermoids in their series, both with an external rhinoplasty approach. The key information necessary for surgical planning is the presence of an intracranial connection to the mass.
Pollock reviewed the surgical treatment of the nasal dermoid cyst and recommended four criteria for a surgical approach. First, the surgical approach should permit access to all midline cysts and should readily permit medial and lateral osteotomies, if required. Second, the surgical exposure should favor the rapid repair of cribriform defects, should they be present, and would permit control of CSF rhinorrhea, if it develops. Third, it would allow reconstruction of the nasal dorsum, if it is required. Fourth, the approach should offer the probability of acceptable scar formation. The approaches recommended are the transverse rhinotomy, the vertical zig-zag rhinotomy, and tripod-eversion rhinotomy. The transverse rhinotomy is used with small to moderately sized lesions with no evidence of intracranial extension. The benefit of this approach is a favorable scar without the splaying which can occur with a vertical rhinotomy. The fistulous opening is excised within a transverse fusiform segment of skin and the tract is cannulated with a lacrimal probe. A second transverse incision is then made over the lower half of the dermoid, and the entire tract is excised. Medial or lateral osteotomies may be performed as necessary for exposure. With larger lesions, especially in the lower two-thirds of the nose a tripod-eversion rhinotomy approach is used.
A transverse incision is made to release the columella, a transfixion incision is made and swept laterally between the upper and lower lateral cartilages. Paraalar incisions will then permit upward rotation of the nose. Any fistulous tract opening is released with a fusiform incision and the tract cannulated with a lacrimal probe. The operating microscope is used to improve visualization. Very large lesions, those in adults in whom underlying bone and cartilage have been damaged by prior surgery or erosion, and in patients with known intracranial extension a longitudinal zig-zag rhinotomy is used for wide exposure. The incisions are designed with limbs extending superiorly at angles greater than forty degrees but less than ninety degrees. Any fistulous opening is excised by fusiform excision. Scar prognosis is better than with a straight incision because the zig-zag are at less than ninety degrees to the relaxed skin tension lines running horizontally across the nose. Rohrich et al recommended an open rhinoplasty approach with a stair-step columellar incision in the majority of cases for the following reasons: ease of exposure, wide exposure of the nasal dorsum, controlled external osteotomies, ease of dorsal reconstruction, and wide exposure of the upper lateral cartilages and septum.
Intranasal lesions are approached via lateral rhinotomy or more recently described by endoscopic techniques. Weiss et al (26) described the use of endoscopic removal of nasal dermoids in two cases. They recommend the use of this technique when the dermoid is located within the nasal cavity and there is little or no cutaneous involvement. This technique can be combined with a small external midline excision of a cutaneous punctum. They recommend the endoscopic technique even with extension to the anterior cranial fossa only recommending a combine intra-extracranial approach when the mass extends to the falx cerebri. Other authors have described excision of nasal gliomas isolated in the nasal cavity without evidence of intracranial connection by imaging.
Midline masses which have an intracranial connection will require a combined approach with a neurosurgeon. A frontal craniotomy is performed, the intracranial portion of the mass is excised and the bone-dura defect is repaired, and the extracranial mass is removed.
Some authors have advocated the use of intraoperative frozen section examination of the stalk of dermoids where it cannot be followed to the base. If only fibrous tissue is found, the stalk is ligated, if dermoid tissue is found an intracranial approach is performed.
Conclusion
The diagnosis of congenital midline nasal masses requires a high index of suspicion. These are rare anomalies, however the life threatening complications of those with an intracranial connection is important to remember. When a patient presents with a mass consistent with nasal dermoid, glioma, or encephalocele, especially a child, biopsy of the lesion should not be performed before imaging is obtained. CT can be used initially with follow up MRI for inconclusive results or evidence of intracranial connection or MRI may be used as the primary imaging study. Treatment of these lesions is surgical excision via an external or intracranial approach or both.
A CASE OF NASOMYIASIS WHOSE AGENT WAS SARCOPHAGA SP.
Introduction
Invasion of tissues and organs in humans and animals is called myiasis. It is known that every fly larva is not a myiasis agent. The flies whose larvae are myiasis agent generally belong to Cyclorrhapha subgroups. Myiasis are classified as obligatory, voluntary and coincidental according to agent character . The fly larvae which case myiasis can live as parasites in skin, subcutaneous tissue, soft tissues, mouth, stomach, intestines, urogenital system, nose, ears and eyes. Myiasis flies are viviparous or oviparous.
Case Report:
The larvae which were seen in our case were examined, and they were identified as “Sarcophaga sp. “. When the literature was reviewed, it was seen that Sarcophaga larvae rarely could cause myiasis in humans and this case was found interesting to be published. A 16-year-old girl was taken to Alasehir State Hospital just after a car accident. She had head trauma, loss of consciousness and pulmonary arrest. She was evaluated as E1M2V1 according to Glaskow Coma Scale and referred to Emergency Unit of Ataturk Training and Research Hospital, Izmır for pulmonary support after being intubated.
She was diagnosed as cerebral confusion and intertrochantric femur fracture after physical examination and laboratory studies were performed in emergency unit, and then hospitalized in Intensive Care Unit of the Anesthesiology and Reanimation Department.
Artificial breathing, free naso-gastric drainage, fluid support with parenteral infusion were applied, and antibiotics, antimucolitic, antiedema and vitamin treatments were given to the patient during the hospitalization period.
Four days after hospitalization , larvae fell down from her nose and she was consulted by Microbiology Department. Physical examination revealed a number of white, moving larvae in her nose. The 16 larvae extracted from her nose were collected in serum physiologic, and examined under the light microscope. It was concluded that these larvae would be agents of myiasis. The studies showed they were second stage Sarcophaga sp. Larvae.
Mechanical cleaning with iodine solution was applied to the patient three times a day for three days. The larvae fall continued by lessening for two days and no larvae was seen during the one month follow-up, but her consciousness status did not show any change.
Discussion
Wohlfahrtia and Sarcophaga larvae generally invade nose or throat directly (7). It is reported that Wohlfahrtia magnifica and more rarely Oestrus ovis larvae cause nasomyiasis in Turkey. Agrawal et.al reported Oestrus ovis larvae as myiasis agent in nasal cavity of a woman (1). A retrospective study of myiasis in 94 Indian children younger than 6-year-old showed that 81 (86.16 %) had ear, 11 ( 11.7 % ) nose and 2 ( 2.12 % ) ear involvement, and September-October period was the most frequent time of the year for infection (10). Yılma et. al. studied 555 heads of adult sheep obtained from southwest France. They were examined for infestation by Oestrus ovis. Infestation was present in 65% of the heads of the adult sheep. The monthly prevalence rate was 44% in April while it was 88.2% in November. This study emphasizes the seriousness of the problem in this region.
It was striking that the larvae in our patient were found in October.
Urinary myiasis, genital myiasis, ophtalmic myiasis, cutaneous myiasis, oral myiasis and rectal myiasis cases which were caused by Sarcophaga larvae have been reported. Jacobsen et. al. reported two patients with hospital-acquired myasis which is a rarely reported nosocomial problem. Both patients were elderly and had lengthy thoracic surgery in August in the same operating room. Larvae, which were of the same species removed from the nares of one patient and from the chest incision of the other. They concluded that Phaenici serricata shuld be presumed as the agent of hospital-acquired myiasis.
The adult forms of Sarcophaga sp. are 13-16 mm in size. Generally, the larvae are left not only on meat or putrid organic materials, but also on human and animal wounds or nose cavities. This species is found in Kazakhistan, Middle Asia, West Europa, North America, Austria, Poland and Turkey.
Like our study, another case from Turkey that; Yazar et al. present a case of oral myiasis in a 15-year-old boy with tuberculosis meningitis. The diagnosis was based on the visual presence of wriggling larvae about 1 cm in size and on the microscopic features of the maggots, especially those relating to stigmatic structures. The larvae were identified as thirdstage larvae of Sarcophaga sp.
In conclusion, in this case we saw that Sarcophaga spp. larvae could cause nasomyiasis and they should be considered in etiologic agents of nasomyiasis.
OLFACTORY PATHWAYS AND LIMBIC SYSTEM
Olfactory Pathways
The sense of smell is much less essential than vision, audition or the somatic senses, and will therefore receive less emphasis in this course. However, since olfactory dysfunction can be an important diagnostic sign, it is important to have at least a rudimentary knowledge of the olfactory pathways.
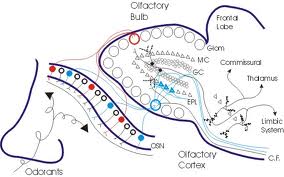
Olfactory Receptors: The olfactory receptors are embedded in a specialized patch of yellow-tinted mucous membrane in the roof of the nasal cavity. These receptors are bipolar neurons covered with modified, non-motile cilia. These cilia probably contain the active sites for the olfactory transduction process. Axons from the olfactory receptors enter small nerve bundles (collectively termed the 1st cranial nerve) which pass through the perforations in the cribiform plate of the ethmoid bone and promptly enter the olfactory bulb. These nerve bundles can be severed as a result of skull fractures or other pathology in this region with a resulting partial or complete anosmia (loss of sense of smell). Much of the sensation we consider to be taste is actually olfactory so patients with anosmia often complain bitterly about loss of pleasure from eating.
Olfactory Bulb: The olfactory bulbs lie on the ventral aspect of the frontal lobes. The olfactory bulbs and all other parts of the olfactory pathways are telencephalic derivatives. Within the olfactory bulbs the olfactory nerves synapse on mitral cells whose axons project directly to the olfactory cortex.
Olfactory Tract:
The olfactory tract connects the olfactory bulb with the cerebral hemispheres. Axons of mitral cells pass directly back to the olfactory cortex on the ipsilateral side. Anterior commissure. This is a small commissure that connects the two halves of the olfactory system. You may want to look for it next time you look at the brain in the lab.
Olfactory Cortex:
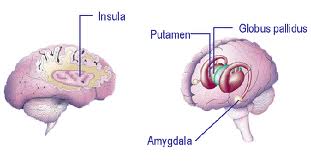
Those portions of the cerebral cortex that receive direct projections from the olfactory bulb (via mitral cell axons) are collectively referred to as the olfactory cortex. Note the olfactory cortex is the one area of cortex that receives direct sensory input without an inter posed thalamic connection. Most of the olfactory cortex is of a primitive 3-layered type. The olfactory cortex is located on the base of the frontal lobe and medial aspect of the temporal lobe. On the base of the frontal lobe it overlies the anterior perforated substance through which the striate arteries enter the interior of the brain (review these arteries in the cortex handout if you have forgotten their significance!). On the temporal lobe the olfactory cortex covers the rostral portion of the parahippocampal gyrus including a medial bulge known as the uncus or uncinate gyrus. The uncus is of clinical significance for two reasons: 1) Seizures often originate in this area (so-called uncinate fits). These seizures are preceded by hallucinations of disagreeable odors, reflecting the olfactory function of the. 2) When the volume of the temporal lobe is increased due to tumors, hemorrhage or edema, the uncus can press against the brainstem and cranial nerves with serious consequences (so-called uncal herniation). The herniating uncus and adjacent part of the parahippocampal gyrus push the brainstem to the opposite side, resulting in damage by pressure against the taut free margin of the tentorium. Thus, brainstem damage is typically contralateral to the side of the herniation. Recall also from Dr. Harting’s lecture that the oculomotor nerve can be damaged on the side that is ipsilateral to the herniation. From the olfactory cortex, olfactory information is relayed via the mediodorsal nucleus of the thalamus to the insular and orbitofrontal cortex. The insular cortex, which is buried in the depths of the Sylvian fissure, also receives taste input from the medial part of VPM and is believed to be the site where olfactory and taste information is integrated to produce the sensation that can be termed flavor. The orbitofrontal cortex on the base of the frontal lobe has an unknown role in olfactory perception.
When testing for olfactory impairment it is necessary to keep two things in mind: 1) The nasal cavities and olfactory pathways up to the level of the anterior commissure are completely separate so each nostril can be tested separately in order to detect a unilateral anosmia; 2) There are endings of the trigeminal nerve (free nerve endings) within the nasal cavity which respond to irritating or pungent odors. Odors of this type must therefore be avoided in testing for anosmia.
Limbic System
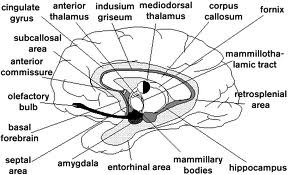
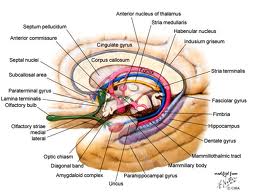
The limbic lobe (from limbus, Lt. = border) is a ring of cortex on the medial aspect of the cerebral hemisphere. The ring of cortex consists of the cingulate gyrus, parahippocampal gyrus, and septal cortex. These 3 cortical areas are connected via the cingulum (Cerebral Cortex handout). The cortical areas within the limbic lobe, together with certain adjacent deep structures, are known as the limbic system. The areas that are usually included within the limbic system are: 1) the limbic lobe, 2) the hippocampal formation and fornix, 3) the amygdala, 4) the septal area, 5) the mammillary bodies (or in some accounts, the entire hypothalamus), and 6) the anterior nuclei of the thalamus. These areas are all closely interconnected by way of one or more of the following important pathways: fornix, mammillothalamic tract, the stria terminalis and the cingulum and appear to function together for the generation of certain emotional and visceral responses as described below. These structures are also sometimes referred to as Papez circuit after the neuroanatomist who pointed out the circular nature of the interconnections.
Many of the limbic structures developed in relation to the olfactory system in primitive vertebrates; hence the term rhinencephalon (literally, “nose brain”) is often used to denote the same areas. In humans, however, most of these areas have little or nothing to do with the sense of smell and the term rhinencephalon seems somewhat misleading (although arguments supporting the use of this term will be found in some textbooks). The anatomy and functions of the different components of the limbic system will first be described individually, followed by a discussion of the system as a whole.
Hippocampal Formation:
The hippocampal formation is a phylogenetically old part of the cerebral cortex located within the temporal lobe. Like the insular cortex, the hippocampal formation has been pushed beneath the external surface of the cerebral hemisphere as a consequence of the overgrowth of the surface area of the neocortex. In cross-section the hippocampal formation presents a complex, folded shape somewhat resembling a seahorse (hippokampus, Gr. = seahorse). The hippocampal formation is actually comprised of several different cortical areas, but we will not consider these differences.
The hippocampal formation projects by way of the fornix to the mammillary bodies of the hypothalamus and the septal nuclei. The fornix is a massive fiber bundle that follows a circuitous course from the hippocampal formation to its target areas. The fibers destined for the fornix collect on the surface of the hippocampus as a thin sheet; they then converge into the fornix proper. The fornix then “pulls away” from the hippocampus and follows a C-shaped course in association with the lateral ventricle. Anteriorally it enters the hypothalamus where it terminates in the mammillary body and septal nuclei.
People with lesions of their temporal lobes involving the hippocampal formation can have a profound disruption of memory function. A severe memory deficit is usually observed only when the damage is bilateral, although it can sometimes occur with one-sided lesions. This will be discussed in some detail in an upcoming lecture. The following is a preview of terms: Neurologists refer to the deficit as a loss of recent memory because memory of recent events is selectively lost. Most of the information that was in long term memory at the time the lesion occurred is retained, but no new information can be added. It seems that the problem lies in the consolidation process of placing new short term memories into long term storage. New information can only be retained for, at most, one or two minutes.
You will also occasionally encounter the terms anterograde amnesia and retrograde amnesia in clinical practice: Anterograde amnesia is the loss of memory of events that occurred after the lesion (equivalent to a recent memory deficit). Retrograde amnesia is the loss of memory of events that occurred before the lesion.
A common cause of bilateral hippocampal damage is anoxia from interruption of blood or oxygen supplies (ischemia or anoxia, respectively). As a result of unique features of its pyramidal cells, the hippocampus is one of the first sites in the brain to be irreversibly damaged from transient ischemia or anoxia. Blood supply to the hippocampus is primarily from branches of the posterior cerebral artery.
A recent memory deficit is also found in Korsakoff’s syndrome, a condition apparently caused by thiamine deficiency associated with alcoholism. Since patients with this problem typically have a grossly visible destruction of their mammillary bodies it has been assumed that these structures are involved in the memory process. Because of the strong connections between the hippocampal formation such a role would not be unexpected. However, it has recently been shown that lesions associated with Korsakoff’s syndrome are not confined to the mammillary bodies but also extend dorsally into the medial part of the thalamus. There is some evidence that without thalamic involvement, no memory deficit is present, although this question is still unsettled. There is a similar controversy concerning the extent to which damage to the fornix affects the memory process. It is clear, however, that many lesions that include the mammillary bodies and/or fornix are associated with a recent memory deficit.
Amygdala:
The amygdala is a relatively large nuclear complex located immediately rostral to the hippocampus, within the temporal lobe. The term amygdala is derived from its almond-like shape (amygdale, GR. = almond). The amygdala is sometimes included with the other nuclear masses of the cerebral hemisphere that are collectively termed the basal ganglia. However, these other telencephalic nuclei are believed to have a motor function while the amygdala is involved in emotional expression and visceral functions. For this reason it is more appropriately considered as part of the limbic system.
The amygdala has been subdivided into many different component nuclei, each with very different connections. You will hear about one of these subdivisions, the central nucleus of the amygdala (CeA. Physiological experiments in animals have revealed that the amygdale receives input from all sensory systems—probably through multisynaptic pathways from the appropriate neocortical areas. The amygdala is connected with the hypothalamus by way of a long, circuitous pathway known as the stria terminalis. The stria terminalis leaves the amygdala caudally and follows a course that approximately parallels that of the fornix. There are also projections from the amygdala directly to the thalamus and neocortex. As in the case of the hippocampus, a great many experimental attempts have been made at discerning the functions of the amygdala. Effects of lesions and stimulation have been examined in animals and humans. There is a great deal of confusion in the literature, but some agreement seems to have been reached that it plays an important role in: 1) control of emotions—especially fear and anger, 2) control of sexual behavior, and 3) control of food and water intake. However, the mechanisms for mediating these behavioral patterns are not well understood.
Before the advent of antipsychotic drugs, bilateral lesions were sometimes deliberately placed in the amygdala. One of the “desirable” effects of this procedure was a docility, where previously violent patients displayed little or no emotion. As described below in connection with Kluver-Bucy syndrome, “naturally-occurring” lesions of the temporal lobes can produce a variety of dramatic changes in emotional expression, some of which presumably result from damage to the amygdala.
Septal Area:
The septal area is a component of the limbic system located anterior to the hypothalamus. It consists of the septal nucleus, the septum pellucidum (thin membrane between the 2 lateral ventricles), and the small portion of neocortex that forms part of the limbic lobe. The septal area is considered to be a component of the limbic system by virtue of its connections with the hypothalamus and hippocampus. The most prominent of these connections is with the hippocampus by way of the fornix. As seen, the fornix gives rise to an offshoot to the septal nuclei on its way to the mammillary bodies. As a result of its connection with the limbic system, including the hippocampus, the septal area is believed to be involved in the control of emotions and, perhaps, memory function. However, our present understanding of its functional role is so limited that you will find no mention of it in Principles of Neurology by Adams and Victor—the “Bible” of neurology.
Cingulate Gyrus:
The cingulate gyrus is the portion of the limbic lobe that overlies the corpus callosum. The cortex of the cingulate gyrus is reciprocally connected with the anterior nuclear group of the thalamus through the thalamic radiations. This connection with the anterior nuclear group puts the cingulate cortex in close communication with the hypothalamus by way of the mammillothalamic tract (see hypothalamus lecture notes). As described above, the cingulate cortex is also connected with the parahippocampal gyrus and septal area by way of the cingulum. The cingulate cortex is believed to be somehow involved in the generation or control of emotional and visceral responses, but as in the case of the septal area, details are unknown. You might be interested in knowing, however, that the cingulate cortex was one of the favorite targets of “psychosurgeons” who attempted to cure or control psychiatric disorders through removal of brain tissue. It was claimed that lesions of the cingulate cortex or underlying cingulum could produce some of the beneficial effects of prefrontal lobotomy without the disturbing side effects (which you will hear about when you discuss Phineas Gage in your small groups).
Kluver-Bucy syndrome:
Bilateral damage to the rostro-ventral portion of the temporal lobes that includes the hippocampal formation, amygdala, and inferotemporal neocortex can give rise to the so-called Kluver-Bucy syndrome. A complete Kluver-Bucy syndrome is only rarely observed, although you will see many patients with certain elements of the problem. As you might expect from the presence of the hippocampal formation in this region, a recent memory deficit is a consistent feature of this syndrome. Other symptoms include visual agnosia (loss of ability to recognize complex visual patterns), and behavioral disturbances including hypersexuality, hyperphagia, and loss of ability to display anger or fear. It has been proposed that the visual agnosia results from damage to the temporal neocortex while the behavioral disturbances result, at least in part, from damage to the amygdala.
Limbic structures as a system:
The behavioral patterns categorized as visceral (e.g., eating, drinking and sexual activity) and emotional are extremely complex and require the coordination of many different kinds of activities. Control of food intake, for example, requires: 1) integration of information from blood levels of metabolites, distention of G.I. tract, etc., to determine when intake of food is necessary (i.e., when a sensation of hunger should be present), 2) initiation of appropriate motor patterns to locate food, 3) analysis of sensory information to identify food, 4) consumption of food, and 5) control of digestion through the autonomic nervous system. A fear response, as another example, requires: 1) analysis of sensory information to determine when the emotional response is appropriate, 2) activation of somatic musculature to produce appropriate movements including flight or attack and characteristic facial expressions, 3) coordination of autonomic effects, i.e., increased pulse rate and respiration, redistribution of blood flow, etc. The complexity of these behavioral responses presumably explains the complexity of the limbic system. Connections with sensory, motor, and autonomic systems are required. In many cases the presence of these connections may give rise to misleading results when different parts of the limbic system are stimulated electrically in an attempt to discern their functions. For example, stimulation of most components of the limbic system produces autonomic effects such as changes in blood pressure and respiration. Similarly, movement can be obtained from stimulation at many points. Finding these effects from stimulation does not mean that the limbic system is primarily involved with autonomic control and movement, but, rather, that it has connections with the hypothalamus and motor areas of the brain for integrating the output of these systems in whatever ways are necessary for the production of visceral or emotional behavioral patterns.
It is worth considering why a single brain “system” is involved with both emotional-visceral and memory functions. One possible explanation should be obvious to anyone having taken an introductory psychology course: emotions and visceral sensations have a strong effect on the learning process. The ability to learn presumably evolved to allow profiting from experience in finding food, mates, avoiding unpleasant or painful stimuli, etc. It is therefore not too surprising that the part of the brain that appears to orchestrate our emotions and regulate visceral functions also plays a central role in learning and memory.
EVALUATION OF CONGENITAL MIDLINE NASAL MASSES
Introduction
Congenital midline nasal masses include nasal dermoids, nasal gliomas, and encephaloceles. These are rare congenital anomalies, estimated to occur in 1:20,000 to 40,000 births. Although rare, these disorders are clinically important because of their potential for connection to the central nervous system. Biopsy of a lesion with an intracranial connection can lead to meningitis or cerebrospinal fluid leak. Preoperative knowledge of an intracranial connection is a necessity to allow for neurosurgical consultation and possible planning for craniotomy. The differential of a midline nasal mass includes inflammatory lesions, traumatic deformity, benign neoplasms, malignant neoplasms, and congenital masses.
Embryology
Knowledge of the normal embryological development of the nose is important to understand the development of congenital midline nasal masses. The development of the nose depends on two crucial steps which occur during the first twelve weeks of fetal development. The first is the formation of the neural tube from the neural fold, which occurs at the dorsal aspect of the embryo between the third and fourth week of gestation. Closure of the neural groove begins in the middle of the embryo and extends in a cranial and caudal direction. The neural tube then gives rise to the neural crest cells. As the neural tube is forming by closure of the neural groove, neural crest cells migrate laterally and anteriorly around the eye to the frontonasal process. In most of the body the neural crest cells are involved in forming ectodermal components, however in the face neural crest cells primarily form mesenchymal cells which provide the bone, cartilage, and muscles of the face. The second crucial step in the development of the nose is the correct migration of these cells into the mesenchyme.
The nose is formed from the frontonasal process and two nasal placodes which develop dorsal to the stomadeum (primitive mouth). The nasal cavity is formed from the invagination of the nasal placodes that appear during the third week of gestation, and is completed around the sixth week, separated posteriorly from the oral cavity by a thin nasobuccal membrane. The two nasal cavities are separated by the nasal septum, which is formed from the migration of neural crest cells into the frontonasal process, growing in an anterior-posterior direction. The ethmoid and sphenoid bones are also formed from the frontonasal process neural crest cells.
The nasal placodes become more prominent and consist of a medial and lateral process. The medial processes approach one another and eventually fuse in the midline. The lateral processes become less prominent as the maxillary process fuses with them. A deep groove in this region, called the nasal-maxillary groove becomes the nasolacrimal duct.
During formation of the skull base and nose, the mesenchymal structures are formed from several centers which will eventually fuse and begin to ossify. Before their fusion there are recognized spaces between these structures which are important in the development of congenital midline nasal masses. These include the fonticulus frontalis, the prenasal space, and the foramen cecum. The fonticulus nasofrontalis is the space between the frontal and nasal bones. The prenasal space is between the nasal bones and the nasal capsule (the precursor of the septum and nasal cartilages). During fetal development these spaces are normally closed by fusion and ossification. Abnormal development of these structures is thought to be involved in the formation of dermoids, gliomas, and encephaloceles of the nose.
Pediatric Anatomic Considerations
Neonates are obligate nasal breathers, due to the high position of the larynx in the neonate and physiologic pattern of breathing and swallowing. The epiglottis abuts the nasal surface of the soft palate, forming an anatomic divide between the airway and digestive systems. Food from the oral cavity is shunted laterally into the esophagus via the pyriform sinuses, whereas air from the nasal cavity is allowed to pass centrally, directly into the larynx. Functionally, this allows the neonate to breathe and feed concurrently. As the child grows, the pharynx elongates and the laryngeal complex descends into the neck, which results in a common pathway that the respiratory and digestive tracts share in the oropharynx. Although oral respiration can now occur, the nasal passages remain the primary airway. It is because of this feature that neonates can suffer from respiratory distress with nasal obstruction.
Evaluation of nasal masses
The evaluation and management of congenital midline nasal masses starts with a complete history and physical exam. Many of these lesions present early in life but adults may also be found who are undiagnosed with these lesions. Dermoids often can present with repeated infection or drainage, a visible sinus tract, and are more solid, noncompressible, and do not transilluminate. Nasal gliomas are also firm, noncompressible, and do not transilluminate but may have overlying telangiectasia. Encephaloceles may be bluish or red, soft, compressible, enlarge with crying, and have a positive Furstenberg test. With intranasal lesions, gliomas arise from the lateral wall while encephaloceles arise more medially. According to Haafiz et al (1995), an intranasal probe can often be passed medial to a glioma but not to an encephalocele. The distinction between glioma and encephalocele is important because while 15% of gliomas have an intracranial connection, all encephaloceles have an intracranial connection.
When a dermoid, glioma, or encephalocele is a suspected diagnosis a biopsy should not be performed before an intracranial connection is ruled out because of the risk of causing meningitis or CSF leak. The majority of these lesions are found in children, and a high index of suspicion is required, especially for a unilateral intranasal mass. The diagnosis is confirmed by CT and/or MRI imaging. Image findings include soft tissue mass, fluid filled cyst, intracranial mass, enlargement of the foramen cecum, and distortion of the crista galli. CT imaging better delineates bony abnormalities while MRI is valuable to identify an intracranial connection. The findings on CT consistent with intracranial involvement are an enlarged foramen cecum or bifidity of the crista galli. Although these findings are consistent with intracranial involvement they are not diagnostic. According to Pensler et al (1988), these findings are only conclusive if they are absent, eliminating an upward intracranial connection. MRI provides better soft tissue detail and ability to visualize in the sagittal plane. Denoyelle (1997) reviewed thirty-six children with nasal dermoid sinus cysts and recommends an MRI scan to confirm any suspected intracranial extension following a CT scan. In his series two patients had false positive CT scan evidence of intracranial connection which was not found at surgery. Another study by Huisman et al (2004) showed that intracranial extension is equally well detected by CT and MRI using indirect imaging signs (bifid or deformed crista galli, widened foramen cecum, defect in cribiform plate) but that with direct imaging signs (identification of intracranially located lesions or sign alterations) MRI was superior, detecting these findings on 2 patients not detected on CT.
Dermoid Sinus Cysts
Dermoid sinus cysts of the nose present as a midline nasal pit, fistula, or infected mass located anywhere from the glabella to the nasal columella, with the distal one-third of the nasal dorsum being the most common site. If composed solely of epidermal tissue, then dermoid cysts are referred to as epidermoid cysts. The lumen of dermoid cysts is made up of a mixture of keratin and lipid. Nasal dermal sinus cysts are firm, non-compressible, non-pulsatile masses, and do not transilluminate. Usually, nasal dermoids terminate in a single subcutaneous tract which can sometimes have hair at the opening. They may secrete sebaceous material or pus, become intermittently inflamed, form an abscess, cause osteomyelitis, broaden the nasal root or bridge, lead to meningitis, or form a cerebral abscess. Connection with the central nervous system has been variably reported to occur. In the case of intracranial extension, the sinus traverses either the cribiform plate or the foramen cecum and is attached to the dura or it can extend in the form of a cyst within the falx cerebri or other brain structures. Faulty closure of the anterior neuropore results in a defect in the anterior fontanelle, foramen cecum, cribiform plate, sphenoid and ethmoid bones. Suspicion of intracranial involvement should remain high.
Associated congenital anomalies occur in 5-41% of cases which include aural atresia, mental retardation, spinal column abnormalities, hydrocephalus, hypertelorism, hemifacial microsomia, albinism, corpus callosum agenesis, cerebral atrophy, lumbar lipoma, dermal cyst of the frontal lobe, coronary artery anomaly, cleft lip and palate, tracheoesophageal fistula, cardiac, genital, and cerebral anomalies. There is no known syndromic association of these anomalies.
A widely accepted theory of dermoid sinus cyst development is the prenasal space theory. According to this theory, during normal development a projection of dura protrudes through the fonticulus frontalis or inferiorly into the prenasal space. This projection normally regresses but if it does not the dura can remain attached to the epidermis and result in trapped ectodermal elements. Thus development of dermoids is hypothesized to result from faulty involution of the dural tract.
Gliomas
Gliomas are made of neuroglial elements consisting of glial cells in a connective tissue matrix with or without a fibrous connection to the dura. There is no fluid filled space connected to the subarachnoid space. These lesions usually present as a red or bluish lump at or along the nasomaxillary suture, or as an intranasal mass. They are characteristically firm, noncompressible, do not increase in size with crying, and do not transilluminate. The overlying skin may have telangiectasias. They can be associated with a widened nose or with hypertelorism secondary to growth of the mass. Intranasal gliomas most often arise from the lateral wall of the nose or less often from the nasal septum. Sixty percent are extranasal, 30% intranasal, and 10% are both. Overall, 15% are connected to the dura. The intranasal type is more often associated with dural attachment (35%) than the extranasal type (9%).
The embryological development of nasal gliomas is similar to nasal dermoids. Thus, if glial tissue is also isolated extracranially by fusion of the cranial sutures, it is hypothesized that a glioma results. Abnormal closure of the fonticulus frontalis can lead to an ectopic rest of glial tissue being left extracranially. This is similar to the mechanism for the formation of encephaloceles, however there is not always an intracranial connection to a glioma and there is always, by definition, an intracranial connection to an encephalocele.
Encephaloceles
Encephaloceles are extracranial herniations of the meninges and/or brain which maintain a subarachnoid connection. If it contains only meninges it is termed a meningocele, when it also contains brain tissue it is called a meningoencephalocele. Thus, when a bony defect allows herniation of dura mater and brain tissue extracranially, an encephalocoele results. Encephaloceles can be divided into three categories: occipital, sincipital, and basal. Occipital are the most common at 75%. Sincipital are frontonasal lesions which present as a mass over the nose, glabella, or forehead. The intracranial connection is usually anterior to the cribiform plate. Basal lesions make up about 10% of lesions and present as an intranasal or nasopharyngeal mass. Basal lesions herniate either through the cribiform plate or posterior to it which explains their presentation in the nose instead of externally.
They enlarge with crying or the Valsalva maneuver. A characteristic sign is the Furstenberg test, which is enlargement with compression of the internal jugular veins. They also can cause a widening of the nose or hypertelorism. Intranasal encephaloceles originate medially in the nasal cavity as opposed to gliomas which most often originate laterally.
The embryologic development of encephaloceles is the same as that for gliomas. Failure of the fonticulus frontalis to close properly can lead to a herniation of intracranial contents which maintains its connection to the subarachnoid space. This connection with the central nervous system and the possibility of containing brain tissue make encephalocele and important entity to rule out when a midline nasal mass is found.
Developmental Considerations to Surgical Planning
Manning et al (2005) described anatomic and developmental constraints that influence treatment options for pediatric patients with skull base pathology depending on the age of the child. They discussed that when considering surgical approaches, one must keep in mind the importance of primary growth centers such as tooth buds, nasal septum and palate, and the zygomatic process of the maxilla. The cranial vault is fairly well developed at birth, but the basicranium and facial skeleton are relatively undeveloped and undergo rapid growth in the first few years of life.
The sinuses are relatively undeveloped at birth and do not afford surgical access to the skull base in the first few years of life (although improving technology is pushing back the minimum age for endoscopic approaches). The ethmoid cells are recognizable by the fifth fetal month and may provide some surgical access to the anterior cranial fossa (with 2.8-mm endoscopes) by age 1 year. Sphenoid pneumatization is highly variable, but the minimum age for an endoscopic optical environment to the middle cranial skull base is probably about age 3 years, on average. The frontal sinuses are usually not radiographically apparent before age 6 to 8 years, and approximately 8% of the population has no significant frontal pneumatization in adulthood.
Maxillary sinus approaches to the pterygomaxillary fossa and middle cranial fossa may be limited by small sinus size and by presence of molar tooth buds before the teenage years. (Manning, 2005).
Surgical Treatment
The treatment of nasal dermoids, gliomas, and encephaloceles is by complete surgical excision. Early surgical intervention is recommended to avoid further distortion of the nose or bony atrophy caused by growth of the mass or recurrent inflammation. The entire lesion along with any fistulous tract must be excised in order to prevent recurrence. The key information necessary for surgical planning is the presence of an intracranial connection to the mass.
Ingraham et al (1943) suggested an external rhinoplasty approach for dermoid lesions that do not extend above the glabella and many surgeons still prefer this approach. The transverse rhinotomy is used with small to moderately sized lesions with no evidence of intracranial extension. The benefit of this approach is a favorable scar without the splaying which can occur with a vertical rhinotomy. The fistulous opening is excised within a transverse fusiform segment of skin and the tract is cannulated with a lacrimal probe. A second transverse incision is then made over the lower half of the dermoid, and the entire tract is excised. Medial or lateral osteotomies may be performed as necessary for exposure.
For high lesions, or those with a small suspected intracranial extension based on imaging studies, a midline incision over the dorsum is preferred, according to Manning et al (2005). For lesions with a stalk extending superiorly, the nasal bones are displaced laterally, and the stalk is dissected from inside the nasal septum. With larger lesions, especially in the lower two-thirds of the nose a tripod-eversion rhinotomy approach is used. A transverse incision is made to release the columella, a transfixion incision is made and swept laterally between the upper and lower lateral cartilages. Paraalar incisions will then permit upward rotation of the nose. Any fistulous tract opening is released with a fusiform incision and the tract cannulated with a lacrimal probe. Microscopic visualization and otologic instruments can be of great value in dissection of these lesions. Small CSF leaks can be managed directly, without a craniotomy. A craniotomy is required for a lesion with extensive intracranial connection, often in a dumbbell configuration with an intracranial cyst above the stalk. With craniotomy approaches to these lesions, however, olfaction must be sacrificed at least on one side.
Meher et al (2005) described a case of the management of a dermoid cyst with intracranial extension, not requiring craniotomy. They described that, after taking neurosurgical opinion, the swelling was excised by a vertical midline incision. The sac of the swelling was found to be going superiorly through a tunnel between the nasal bones and the underlying nasal septum. The nasal bones were removed along with the adjacent anterior part of the frontal bone. Intracranial extension through the cribiform plate was identified. The wall of the sac was incised and, after evacuating the contents, it was removed except for its base where it was attached to the dura. The secretory epithelial surface of the remnant of the sac was destroyed by bipolar electrocautery. The nasal bones were replaced and the wound was closed in layers. They stated that the postoperative period was uneventful and that there was no recurrence during a two year follow-up period.
Manning et al (2005) mention that recent reports in the literature show that an endoscopic intranasal approach for intranasal gliomas even with a central connection is possible, assuming surgeon experience with endoscopic repair of small CSF leaks, this includes studies by (Yokoyama et al (1999), Rahbar et al (2003), and Agirdir et al (2004). This is also limited by the age of the patient. Approach for surgical excision is based on imaging diagnosis of possible CSF connection, location of the pathology, and surgeon experience.
Intranasal lesions are approached via lateral rhinotomy or more recently described endoscopic techniques. Weiss et al (1998) described the use of endoscopic removal of nasal dermoids in two cases. They recommend the use of this technique when the dermoid is located within the nasal cavity and there is little or no cutaneous involvement. They recommend the endoscopic technique even with extension to the anterior cranial fossa only recommending a combine intra-extracranial approach when the mass extends to the falx cerebri. Other authors such as Burkhardt et al (1999) and Dimov et al (1989) have described excision of nasal gliomas isolated in the nasal cavity without evidence of intracranial connection by imaging.
Sincipital (anterior or frontoethmoidal) encephaloceles have a facial component and typically require a combined approach with craniotomy for repair and reconstruction. The rare basal encephaloceles present as a nasal mass with the potential for airway obstruction or meningitis. The two most common imaging findings with basal encephaloceles are foramen cecum defects with variable extension into the ethmoid roof and cribiform or isolated ethmoid roof defects with low-lying funnel-shaped anterior skull base anatomy (Woodsworth et al, 2004).
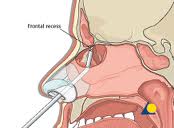
Traditional approaches to basal encephaloceles involved an anterior craniotomy with repair of the skull base defect with pericranial flaps. Management is shifting in many centers to endoscopic nasal approaches even for large encephaloceles in young patients (Marxhall et al, 2001). The encephalocele itself tends to create more intranasal space by deflecting the septum and middle turbinate, although angled telescopes, possibly including 70° scopes, may be necessary to visualize anterior connections. Generally, the intranasal portion of the encephalocele is ablated progressively with bipolar cautery until the skull base defect is seen. The mucosal cuff is removed circumferentially around the bony defect, which is then repaired endoscopically.
Conclusion
Congenital midline nasal masses include nasal dermoids, nasal gliomas, and encephaloceles. These are rare congenital anomalies, estimated to occur in 1:20,000 to 40,000 births. Although rare, these disorders are clinically important because of their potential for connection to the central nervous system. Biopsy of a lesion with an intracranial connection can lead to meningitis or cerebrospinal fluid leak. The treatment of these masses is surgical excision. Preoperative knowledge of an intracranial connection allows for neurosurgical consultation and planning for craniotomy. Surgical strategy depends on the location and extent of the lesion, ranging from local excision via an open or endoscopic approach, to a combined intracranial-extracranial approach.
PEDIATRIC ENDOSCOPIC SINUS SURGERY
Purpose
Sinus disease is a very common source of morbidity for many children. On average, children average 6-8 upper respiratory tract infections per year. 5-13% of all URIs are complicated by secondary bacterial infection of the paranasal sinuses. Young children are unable to communicate some of the symptoms of sinusitis such as headache, nasal obstruction, and sinus pain/pressure. This may pose a diagnostic challenge to clinicians who must rely on parental reporting of symptoms and physical findings. The purpose of this Grand Rounds is to explore the evidence available investigating pediatric FESS to determine its indications, safety, and long-term efficacy. A focus is kept on the management of chronic rhinosinusitis in the pediatric population.
History
Pediatric endoscopic sinus surgery was first performed in the late 1980s with reported short term success over 80%. The initial surgical indications were broad. Indications for adult FESS were sometimes used in the pediatric population, without evidence-based data. Initial studies of pediatric FESS were often retrospective, without a comparison to medically treated or non-treated group. A paradigm shift occurred when prospective studies indicated that medical therapy was an effective approach to treatment in chronic rhinosinusitis (CRS) in the pediatric population. Additionally, research in animals revealed that sinus surgery may have a significant effect on the developing facial skeleton. Recent studies have conducted using an evidence-based approach to pediatric sinus disease that includes FESS as an option.
Indications for Endoscopic Sinus Surgery in Children
In 1998, a consensus panel in Belgium determined guidelines for FESS in children. There were 9 indications listed: complete nasal obstruction in cystic fibrosis caused by massive polyposis or closure of the nose by medialization of the lateral nasal wall, antro-choanal polyps, intracranial complications of sinus disease, mucoceles and mucopyoceles, orbital abscesses, traumatic injury to the optic canal, dacrocystorhinitis secondary to sinusitis, fungal sinusitis, and some meningo-encephaloceles. A possible indication listed was CRS refractory to medical management. An additional indication found in recent literature utilizing FESS is for surgical resection of anterior skull-base tumors including juvenile nasopharyngeal angiofibroma.
CRS in Children
CRS was listed as a possible indication for pediatric FESS in the consensus meeting. CRS usually carries a multifactorial etiology. This disease process is due to an insult to normal drainage pathways of the paranasal sinuses leading to stasis of secretions and secondary overgrowth of microorganisms. Recurrent URIs change the normal mucosal thickness as well as impairing normal mucocilliary clearance of sections. In the daycare setting or in environments with multiple children, the pediatric population is at risk of acquiring recurrent URIs. Additionally, children raised in households that smoke tobacco tend to have greater incidences of sinus and middle ear disease.
Allergic rhinitis, asthma, aspirin allergy, and atopy may contribute to the development of CRS. Allergic rhinitis is reported to be present in up to 40% of people at some point in childhood. It is also associated with up to 80% of cases of CRS. Allergic rhinitis, asthma, aspirin, and atopy may be present in the family of children with CRS. Additionally, serologic or skin testing for potential allergens should be considered in all children with CRS.
Adenoid hypertrophy may obstruct the nasopharynx, thus preventing the normal clearance of secretions resulting in stasis and possible infection. Structural abnormalities, such as choanal stenosis/atresia, severely deviated septum, large obstructive agger nasi air cells, hypoplastic maxillary sinuses and bony remodeling due to active sinus disease processes may obstruct normal clearance of secretions. A CT scan of the sinuses may be helpful in addition to endoscopic findings in diagnosing these structural abnormalities.
Gastroesophageal reflux disease (GERD) has also been associated in children with CRS. One study discovered that 19 of 30 patients with CRS had tested positive for GERD by pH probe study. 79% of these patients showed improvement after medical and behavioral therapy for reflux. Another study found that 25 of 28 children who were candidates for FESS due to sinusitis were able to avoid surgery with a regimen of a proton-pump inhibitor (PPI) and behavior modification. Empiric therapy with a PPI with or without a prokinetic agent and behavior modification is an acceptable approach for treating children with CRS suspected to be due to reflux.
Immunologic deficiency, cystic fibrosis, and ciliary dyskinesia pose a significant problem to treatment of CRS. Recurrent and chronic infections that respond poorly to medical therapy should warrant further immunologic workup in a child. Quantitative (antibody titers) and qualitative (T-cell function) immunologic testing should be considered in these children. Additionally, recurrent upper and lower respiratory tract infections should lead to further testing. A mucosal ciliary biopsy may be necessary to diagnose ciliary dyskinesia. A sweat chloride test should be performed in all children with sinonasal polyps to investigate for cystic fibrosis.
Allergic fungal sinusitis (AFS) is a unique pathologic process that may result in severe sinus disease. It is caused by a hypersensitivity response to fungi in the paranasal sinuses. Apergilles, Alternaria, Bipolaris, Culvularia, and Drechslera are some of the common fungi known to cause AFS. In addition to the sinus symptoms of CRS, children may have facial abnormalities such as proptosis due to bony remodeling of the facial skeleton. Charcot-Leydon crystals, degranulated eosinophils, and the presence of fungal hyphae are diagnostic findings on micoscopic examination of the (“peanut butter”) sinonasal debris or allergic mucin removed from patients with AFS. CT findings may reveal significant sinonasal obstruction with double-density and significant bony remodeling with possible cranial or orbital extension.
Acute Bacterial Sinusitis
In order to understand CRS, the management of acute sinusitis must be discussed. In 2001, Clinical Practice Guideline for the management of sinusitis in children was published in Pediatrics. Different forms of sinusitis were also defined:
- Acute bacterial sinusitis (ABS): bacterial infection of the paranasal sinuses lasting less than 30 days in which symptoms resolve completely.
- Subacute bacterial sinusitis: bacterial infection of paranasal sinuses lasting between 30-90 days in which symptoms resolve completely.
- Recurrent acute bacterial sinusitis: episodes of bacterial infection of the paranasal sinuses, each lasting less than 30 days and separated by intervals of at least 10 days during which the patient is asymptomatic.
- Chronic sinusitis: episodes of inflammation of the paranasal sinuses lasting more than 90 days. Residual respiratory symptoms persist such as rhinorrhea, nasal obstruction, or cough.
- Acute bacterial sinusitis superimposed on chronic sinusitis: patients with residual respiratory symptoms develop new respiratory symptoms. When treated with ntimicrobials, the new symptoms resolve, but underlying residual symptoms persist.
The recommendations made in this guideline were for management of ABS. Antibiotics were recommended to achieve a more rapid clinical cure. Children with uncomplicated ABS mild to moderate severity not attending daycare are recommended to be treated with either amoxicillin 45 mg/kg/d in 2 divided doses or 90 mg/kg/d in 2 divided doses. For penicillin allergic patients, cefdinir (14 mg/kg/d in 1-2 doses), cefuroxime (30 mg/kg/d in 2 doses), cefpodoxime (10 mg/kg/d 1 dose), clarithromycin (15 mg/kg/d 2 doses), or azithromycin (10 mg/kg/d on day 1, and 5 mg/kg/d for 4 days) are recommended.
If symptoms are severe, or refractory usual amoxicillin or other antimicrobial, or daycare is attended high-dose amoxicillin-clavulinate (80-90 mg/kg/d in 2 doses) or IM ceftriaxone (50 mg/kg single dose) followed by oral therapy is recommended. Duration of therapy may be 10, 14, 21, or 28 days but an alternative suggestion is 7 days of therapy beyond resolution of symptoms.
For cases of failure of cure following oral antibiotics, IV cefotaxime or ceftriaxone are recommended. A maxillary sinus aspiration may also be appropriate to determine the microbial species present and determine the antibiotic sensitivities. Children with complicated or suspected complications of ABS should be treated promptly and aggressively and have appropriate consultations with an otolaryngologist, infectious disease specialist, ophthalmologist, and neurosurgeon. IV ceftriaxone (100 mg/kg/d in 2 doses) or ampicillin-sulbactam (200 mg/kg/d in 4 doses) should be started empirically and a maxillary sinus aspiration should be attempted. Vancomycin (60 mg/kg/d in 4 doses) may be used for cases of suspected methicillin-resistant Staphylococcus aureus or for patients with severe penicillin allergy. A CT scan is also recommended to determine possible intracranial or orbital involvement such as an epidermal or subperiosteal abscess which may not be easily diagnosed on physical findings of a young ill child.
The Role of Antibiotics in CRS
In 2001, Don published a study that recommended a stepwise protocol for the management of CRS in children. The purpose of this study was to evaluate the efficacy of IV antibiotics for treatment of CRS in children. This was a retrospective study of 70 patients with a diagnosis of CRS without cystic fibrosis, immunologic deficiencies, nor facial anatomic abnormalities with ages ranging from 10 months to 15 years. All patients had at least 12 weeks of CRS symptoms with persistent sinus disease present on CT after 3-4 weeks of oral antibiotics. All patients underwent maxillary sinus aspiration and irrigation with selective adenoidectomy depending on presence of adenoid hypertrophy on CT or intraoperative findings during maxillary sinus aspiration and placement of long-arm IV catheter. All patients also underwent a 1-4 week course of (culture-directed when possible) IV antibiotics. Interestingly, 73% of patients had at least one organism present on culture with H. influenzae being the most common pathogen present. 43% of patients had multiple organisms on aspiration. The antibiotics used for treatment were cefuroxime (43%), Unasyn (31%), ticarcillin with clavulanate (21%), ceftriaxone (3%), and vancomycin (1%). 66% of patients also had a course of oral antibiotics following completion of IV therapy. 10% of patients were reported to have relatively minor complications without any mortality.
An important finding of this study was that 89% of patients had initial improvement after IV antibiotic therapy. Of these, 74% had long term follow-up between 6 to 62 months (mean 25 months). In this group with long term follow-up, 88% were reported to have long term improvement by the parents of the children. 12% did not have long-term improvement, but also did not require FESS. In the group with long term follow-up, 23% had no further episodes of sinusitis, whereas, 77% had subsequent episodes of sinusitis, but were reported to be completely resolved following oral antibiotic therapy. There was no reported difference in this group with improvement treated with concomitant adenoidectomy versus without adenoidectomy. 11% of patients in this study did not have initial improvement following IV antibiotics and required FESS. Of those requiring FESS, 88% had long-term follow-up. 43% had long-term improvement.
A protocol was proposed by Don et. al from this study for children with CRS with symptoms 12 weeks duration or longer refractory to 3-4 week treatment with oral antibiotics. First, they recommended an allergy and immunology assessment with appropriate medical management for those found to have abnormalities. For patients who were found to have no evidence of allergic or immunologic disease and for patients refractory to medical management of these processes, a CT scan of the paranasal sinuses was deemed appropriate as the next step.
For those with an anatomic abnormality leading to sinus pathology, FESS was proposed. For those with positive findings of sinusitis without specific anatomic abnormalities, bilateral maxillary sinus lavage with culture directed IV antibiotics and selective adenoidectomy was proposed. If there was no improvement, FESS was then deemed necessary.
This study indicated that medical management of pediatric CRS refractory to oral antibiotics is effective and relatively safe. FESS may be reserved for those patients refractory to IV antibiotics. However, this study also had some limitations. It was a retrospective review of patients and there was no stratification for severity of symptoms. The questionnaire used in this study was not reported to be a validated questionnaire for reporting symptoms by the parents of the children investigated. Additionally, there was no standardized analysis of the CT findings of the patients. The role of adenoidectomy could not be assessed in this population as another therapeutic option in the management of CRS in children. Lastly, the role of topical steroids, saline irrigations, and antihistamines was not assessed. The data from this study is nonetheless quite useful in attempting to manage CRS in children.
The Role of Adenoidectomy in CRS
Adenoid hypertrophy is not an uncommon finding in children. Adenoid hypertrophy may lead to obstruction of the Eustachian tube as well as obstruction of the nasopharynx resulting in stasis of paranasal sinus secretions. Additionally, adenoid tissue has been found to be a reservoir for pathogenic bacteria. Stasis of secretions along with the presence of bacteria may result in sinusitis. Previous studies have indicated the efficacy of adenoidectomy in the treatment for chronic otitis media with effusion. Additionally, the overall success rate for adenoidectomy in the treatment of CRS in children has been documented to be about 50%.
A study was conducted by Ramadan in 2004 to evaluate the success of FESS alone, adenoidectomy alone, and FESS with adenoidectomy as surgical options for children with CRS refractory to medical management. This was conducted as a prospective non-randomized study over 10 years and follow-up assessment at 12 months. The results of this study were that success rates were 87.3%, 75%, and 51.6% in the FESS with adenoidectomy, FESS alone, and adenoidectomy alone groups, respectively. Revision rates were 7.6%, 12.5%, and 25%, respectively. Multivariable analysis was performed using logistic regression model.
The success rate of surgery was noted to be 59.5% for children 6 years or younger. This was significantly lower that children over 6 years who had a success rate of 84%. Children over the age of 6 years who underwent FESS with adenoidectomy had the best success rate 96%, which was significantly higher than FESS alone (79%) and adenoidectomy alone (67%). Success rates were not significantly different for FESS with adenoidectomy (76%), FESS alone (67%), and adenoidectomy alone (44%) in children 6 years and under. In asthmatics within this study (43.2%), success after surgery was found to be 62% compared to 80% for children without asthma. The success rate for asthmatics that underwent FESS with adenoidectomy (82%) was not significantly different from FESS alone (77%), but both groups were significantly better than adenoidectomy alone (37%). In non-asthmatics, success with FESS with adenoidectomy was 90% but this was not significantly different from the FESS alone group (79%), but was significantly better than adenoidectomy alone group (65%). For the children exposed to smoke (27%), success after surgical therapy was not significantly different from the group not exposed to smoke, 67% and 74%, respectively. For children exposed to smoke, FESS with adenoidectomy had greater success (82%) compared to FESS alone (64%) and adenoidectomy alone (46%). An additional variable analyzed in this study was severity of disease based upon Lund-McKay CT score. For patients with Lund McKay score greater than 4, they had greatest success when FESS with adenoidectomy was performed compared to FESS alone and adenoidectomy alone (87%, 72%, and 46% respectively). In children with scores of 4 or less, there was no significant difference (90%, 100%, and 59%). 47% of children in this study had allergies, and overall success after surgery for those with allergies was similar to those without allergies (74% and 71%, respectively). There were few surgical complications. 2.9% had minor orbital complications involving orbital entry and postoperative orbital ecchymosis without any complaints. No postoperative CSF leaks, bleeding, or lacrimal duct injuries were found.
This study provided an insight into the best surgical intervention to perform in children with CRS refractory to medical management based upon severity of disease and age. Children with asthma exposed to smoking had least benefit from adenoidectomy alone, but a greater success when FESS was performed in addition to adenoidectomy. Children over 6 years with Lund-McKay score greater than 4 had a better outcome when FESS with adenoidectomy was performed. A conservative approach for children age 6 and younger with a Lund-McKay score 4 or less without asthma may be with an adenoidectomy alone as the initial procedure.
Quality of Life After Surgical Therapy for Sinus Disease
A prospective, nonrandomized quality of life (QOL) study was conducted by Rudnick in 2006 using the validated SN-5 QOL survey completed by caregivers of children following surgical therapy for sinonasal disease. Adenoidectomy (59%) and FESS (41%) were performed in these children with preoperative SN-5 assessment as well as second assessment 6 months after surgery. This study revealed that all children had significant improvement after surgical intervention and there was no significant difference in QOL scores between children undergoing adenoidectomy versus FESS.
Accuracy of CT Finding in CRS in Children
Although CRS is a clinical diagnosis, CT findings are important in diagnosis. In 2004, Bhattacharyya et al. conducted a prospective study to determine the diagnostic accuracy of CT in pediatric CRS using the Lund-McKay score. Two cohorts were followed: one undergoing a preoperative CT for planning of FESS with known cystic fibrosis or anatomic abnormalities, and the other group undergoing CT for non-sinusitis reasons. This study revealed an average Lund-McKay score of 10.4 in the group with sinus disease and 2.8 in the group without clinical sinus disease. This revealed that incidental paranasal mucoperiosteal thickening in the absence of clinical symptoms is present in the pediatric population. When a score of 5 is used to represent true sinus disease, CT scan demonstrated a sensitivity of 86% and specificity of 85%.
CRS, Age and Sinus Surgery
In order to understand the appropriate management of CRS in children, recognition of successful surgical management and age at intervention must be discussed. A cohort study was performed by Ramadan in 2003 to determine the relationship of age to outcome after FESS in children. A questionnaire was completed by caregivers 12 months after FESS with selective adenoidectomy for CRS for assessment of symptoms. This study revealed that children 6 years and older had 89% success, whereas, children under 6 years had 73%. Additionally, 9% that required revision surgery were under 6 years. After age stratification, children under 4 years had 35% success, children 4-8 years had 88% success, and children over 8 years had 86% success. This study also found that children under 3 years had the highest failure rate with 75% requiring revision surgery. Some of the results of this study are difficult to apply to a larger population as there was a small patient population in the younger age groups. However, this study does indicate that older children tend to have better outcomes after FESS compare to younger children with CRS. This may provide insight into management of younger children with perhaps more emphasis on medical therapy.
FESS and Facial Growth
During the early years of pediatric FESS, concern was raised regarding the effects of surgery on the developing facial skeleton. Animal studies indicated significant detrimental effects when sinonasal surgery was performed on young rabbits and piglets. In one study, unilateral sinus surgery was performed on piglets with subsequent evaluation of development based on CT finding. This study revealed that on the operated side, maxillary and ethmoid sinuses only reached a fraction the size of the non-operated side (57% and 65%, respectively). Earlier studies of cleft palate surgery and its effects on maxilla growth revealed a greater incidence of midfacial maldevelopment after cleft palate repair. Another study of pediatric mandibular fractures revealed significant asymmetry after repair in children.
Wolf published a study of 124 children treated with FESS for CRS revealing no clinically significant disturbance in facial bone development. However, only 4% of patients were under 5 years during the most rapid period of growth of the paranasal sinuses. In 1996, a study revealed that maxillary sinus hypoplasia may be a consequence of endoscopic sinus surgery, but there was no clinically apparent facial asymmetry.
A study by Senior et al. in 2000 evaluated the quantitative impact of pediatric sinus surgery on facial growth and development. The study group consisted of children requiring unilateral sinus surgery for periorbital or orbital sinusitis. The control groups were adults without evidence of sinusitis on CT and adults with findings of sinusitis on CT and clinical history of childhood sinus symptoms. This study had a mean follow-up of 6.9 years and found that there was no significant difference in sinus volumes amonght the groups.
A well-known study by Bothwell et al. in 2002 investigated the long-term outcome of facial growth after FESS. This was a retrospective study utilizing anthopometric analysis of 12 facial parameters and qualitative analysis of Caucasian children diagnosed with CRS who underwent FESS versus children with CRS who did not have FESS and normal controls. 10 year follow-up was reported in the study. The Pediatric Rhinosinusitis CT Scoring System was used to determine severity of disease based upon CT findings. The results of this study revealed no significant difference for anthropometric measurements among the groups. Interestingly, on qualitative evaluation by a blinded observer, the non-surgical group had a lower score than the group treated with FESS.
A recent prospective study by Peteghen et al. in 2006 revealed that there was no significant difference in cephalometric parameters among children with cystic fibrosis who underwent FESS compared to normal age-matched controls. These studies indicate that FESS does not significantly alter the normal growth and development of the pediatric facial skeleton. However, it is important to understand that FESS during rapid growth of the facial skeleton had not been thoroughly investigated, and may result in asymmetry.
Safety and Efficacy of Pediatric FESS
Surgical interventions in the pediatric population must be safe and effective to be appropriate option. The safety and efficacy of FESS in adults is well-documented. In 1998, a meta-analysis was published by Hebert investigating the outcomes of FESS. 8 articles with 832 patients met the inclusion criteria, and the publisher included 50 previously unpublished patients from the home institution. A positive outcome of FESS was found to be 88.7% with a mean follow-up of 3.7 years. Additionally, the major complication rate was found to be 0.6% in 6 of the 8 articles included. Blood transfusion requirement and meningitis were the reported complications.
Image-Guided Pediatric FESS
With the advent of image-guided surgery, the ability to perform complex and delicate procedures in the paranasal sinuses has been enhanced. Identification of obscured landmarks may be difficult in disease states. Indications for computer-assisted endoscopic sinus surgery are revision surgery; distorted anatomy of development, postoperative, or traumatic origin; extensive sino-nasal polyposis; pathology involving the frontal, posterior ethmoid, and sphenoid sinuses; disease abutting the skull base, orbit, optic nerve, or carotid artery; cerebrospinal fluid rhinorrhea or conditions where there is a skull-base defect; benign and malignant sino-nasal neoplasms; and choanal atresia. In children, due to smaller and less distinct structures that may not provide adequate identification of critical structures such as the lamina papyracea and skull-base, image-guidance is an asset to surgeons.
Conclusion
Pediatric sinus disease is sometimes a challenging process for clinicians. Patients, parents, and caregivers seek medical assistance for management of sinusitis. The role of the clinician is to provide the best recommendations and options available based upon the evidence presently available. Pediatric FESS has undergone much debate in the otolaryngologic society. Questionnaires sent to pediatric otolaryngologists provide an insight into the contemporary management trends for this process, but do not necessarily reflect the data available in studies. Further research in this area is needed to understand the long-term success and a more comprehensive understanding of when FESS should be performed in the setting of CRS.
THE MIKULICZ CELL IN RHINOSCLEROMA
 The stages in the development of the Mikulicz cell in human rhinoscleroma were studied in biopsy specimens obtained from 10 patients using light, immunofluorescent and electron microscopy. The Mikulicz cell was identified morphologically as a macrophage, not a plasma cell. Acutely inflamed areas of rhinoscleroma presented abundant bacteria with a slime layer. The microorganism was infrequent and the mucopolysaccharide was scanty in rhinoscleromal tissue, where plasma cells predominated, and in cicatricial fibrous tissue. In the granulomatous stage of rhinoscleroma, the mucopolysaccharide was found within the Mikulicz cells. The vacuoles observed in the Mikulicz cells were considered to be phagosomes containing, principally, bacterial mucopolysaccharide and few bacteria and, to a lesser extent, swollen mitochondria. It was concluded that the slime layer of Klebsiella rhinoscleromatis plays an important role in the pathogenesis of the disease. It is postulated that this material is a nondigestible mucopolysaccharide that resides in the phagosomes of macrophages, increases the osmotic pressure and forms multiple hydropic vacuoles that rupture not only the phagosomes but also the cells, resulting in the liberation of the mucopolysaccharide. This would initiate a cycle that would prolong the disease in the absence of the bacteria (Am J Pathol 73:47-58, 1973).
The stages in the development of the Mikulicz cell in human rhinoscleroma were studied in biopsy specimens obtained from 10 patients using light, immunofluorescent and electron microscopy. The Mikulicz cell was identified morphologically as a macrophage, not a plasma cell. Acutely inflamed areas of rhinoscleroma presented abundant bacteria with a slime layer. The microorganism was infrequent and the mucopolysaccharide was scanty in rhinoscleromal tissue, where plasma cells predominated, and in cicatricial fibrous tissue. In the granulomatous stage of rhinoscleroma, the mucopolysaccharide was found within the Mikulicz cells. The vacuoles observed in the Mikulicz cells were considered to be phagosomes containing, principally, bacterial mucopolysaccharide and few bacteria and, to a lesser extent, swollen mitochondria. It was concluded that the slime layer of Klebsiella rhinoscleromatis plays an important role in the pathogenesis of the disease. It is postulated that this material is a nondigestible mucopolysaccharide that resides in the phagosomes of macrophages, increases the osmotic pressure and forms multiple hydropic vacuoles that rupture not only the phagosomes but also the cells, resulting in the liberation of the mucopolysaccharide. This would initiate a cycle that would prolong the disease in the absence of the bacteria (Am J Pathol 73:47-58, 1973).
Rhinoscleroma is a progressive, granulomatous infectious disease, usually limited to the upper respiratory tract and produced by Klebsiella rhinoscleromatis (Klebsiellae tribe III) .1,2 Sporadic cases and endemic foci of the disease have been found in 68 countries. Clinically, the disease has been described in progressive stages; however, overlapping of the stages occurs frequently in the individual patient. The first stage is the rhinitic (cataharral, mucopurulent) or atrophic stage, where the histopathology is nonspecific. Usually there are abundant polymorphonuclear leukocytes and cellular debris; a meticulous examination may also reveal some Mikulicz cells and plasma cells. The second stage is the infiltrative (nodular, granulomatous) or florid stage, in which Mikulicz cells are abundant and a characteristic infiltrate of plasma cells with prominent Russell bodies (Cornil or Mott cells), lymphocytes and foci of polymorphonuclear leukocytes also appears. Granulation tissue rich in blood vessels is also present in this stage. The third stage is the cicatricial, fibrous or scarring stage, in which connective tissue is prominent, while Mikulicz cells and plasma cells are rare. Much of the enigma concerning the relative importance of the bacteria and the tissue response in the development of this chronic disease concerns the Mikulicz cell. This cell has been described as a large, foamy, mononuclear cell containing numerous gram-negative bacilli and is considered to be characteristic of the disease. The pathogenesis of the Mikulicz cells remain uncertain.’)”” Some investigators have suggested that this cell arises from plasma cells,”1 while others have suggested that this cell is a macrophage.” The present morphologic study of proven cases of human rhinoscleroma, using light, electron and immunofluorescent microscopictechnics, concerns the pathogenesis of this peculiar cell, the Mikulicz cell.
Materials and Methods
Nasal biopsies were obtained from 10 patients with rhinoscleroma in different stages of evolution, diagnosed by histologic and bacteriologic criteria. The tissue was processed for light, fluorescent and electron microscopic studies. The patients were from the clinic and hospital of the Universidad del Valle in Cali, Colombia, an area of endemic rhinoscleroma. Light Microscopy Specimens were fixed in 10% buffered formalin, embedded in paraffin; 5-t thick sections were stained with hematoxylin and eosin, periodic acid-Schiff (PAS) and Warthin and Starry stain for bacteria. Sections 1 Mt in thickness were embedded in Maraglas (see electron microscopy below) and stained with Paragon Multiple Stain (Paragon, C. and C. Co., Inc., New York) for examination by light microscopy.
Fluorescent Microscopy
The procedures have been reported in previous publications and are described here briefly. Antibodies against K rhinoscleromatis were prepared in rabbits by using the whole microorganism mixed with complete Freund’s adjuvant. Globulins were separated from the serum with half-saturated ammonium sulfate and labeled with fluotescein isothiocyanate. The conjugates were absorbed in rat liver powder, and pure cultures of strains of K pneumoniae were grown. Five-mic-ron sections of biopsy tissues were “stained” by direct fluorescent antibody technic. A Zeiss fluorescent microscope was used and photographs were taken on Ektachrome film (Eastman Kodak Co, Rochester, NY).
Electron Microscopy
Fragments of tissue 1 to 2 mm in diameter were fixed for 2 hours at 4 C in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.20) containing one drop of of 1.0% CaC/10 ml of fixative. Tissues were washed ovemight in the same cold buffer with 5% sucrose added, postfixed in 1% osmium tetroxide in phosphate buffer for 1 hour and then dehydrated in a graded series of cold ethanol, followed by propylene oxide and embedded in Maraglas. Thin sections were cut with a diamond knife, stained with uranyl acetate and lead nitrate and examined in a Phillips EM 300 or an RCA EMU-4 electron microscope.
Results
All specimens examined contained Mikulicz cells described below. In the following sections the morphology of rhinoscleroma is limited to these cells without repeating established observations.
Light Microscopy
The Mikulicz cell was identified as a large (10 to 100 p ) mononuclear cell with an irregular outline. The cell was sometimes fragmented and difficult to delineate from other cells. WVith hematoxylin and eosin staining, the cytoplasm in the well-developed large Mikulicz cell was vacuolated and frequently contained a single, irregular vesicle with granular and fibrilar debris. Coalition of the vacuolated cells was frequent, with formation of multicellular microcysts. The smaller, less altered Mikulicz cells, usually localized around blood vessels, contained few vacuoles, had a granular cytoplasm and an oval beanshaped nucleus, sometimes with an identation. With PAS staining, slightly positive material could be seen within the majority of the vesicles and was concentrated in the periphery of these structures. With Warthin and Starry staining, bacteria could be demonstrated in some of the vacuoles and microcysts. The bacteria were more abundant in areas of acute inflammation. In thick sections of Maraglas-embedded tissues, multiple vesicles could be seen in the cytoplasm of Mikulicz cells with well-preserved intervesicular bridges containing granules. Some nuclei were stellate and some were pyknotic; in some cells nuclei were absent. In the granulomatous stage of rhinoscleroma, Mikulicz cells were abundant but less numerous in places of heavy plasma cell infiltration and vice versa. In the cicatricial areas, occasional Mikulicz cells were present in the middle of bundles of collagen fibers. In each case, especially where large or multiple biopsies had been taken, it was possible to recognize areas peculiar to each of the so-called stages of the disease.
Fluorescent Microscopy
Granular fluorescent material of nonuniform size was demonstrated in Mikulicz cells, multicellular microcysts and interstitial tissues. This fluorescent material was condensed at the periphery of the vesicles and was present in the majority of these vacuoles. Bacteria were demonstrated in a limited number of the vacuoles of the Mikulicz cell and microcysts. In the areas of acute inflammatory infiltration the fluorescent material was more abundant and the bacteria were more numerous; both fluorescent material and bacteria were located in the interstitial tissues and within the cells. In the granulomatous areas, the fluorescent material was limited to the vacuoles of the Mikulicz cells and to microcysts, very little appeared in the interstitial tissues. The fluorescent material appeared to be less abundant in the granulomatous areas with heavy plasma cell infiltrates. In the fibrous areas, occasional Mikulicz cells contained a few fluorescent granules and very rarely bacteria.
Electron Microscopy
The cell boundaries of the Mikulicz cell were usually difficult to see, and many of the cells appeared fragmented. The organelles in the cytoplasm were located in the intervesicular bridges and presented a variety of alterations according to the extent of the vacuolization. Mitochondria were usually oval or round with marked vacuolization and loss of cristae. The dense bodies of these organelles were sometimes very prominent. The smooth endoplasmic reticulum was not prominent, especially in the well-developed Mikulicz cell. The Golgi apparatus and primary lysosomes were seldom visualized. Rough endoplasmic reticulum and isolated ribosomes were sometimes found in the intervacuolar bridges. Smooth membrane vesicles of different sizes were numerous; in the majority of cases, they were interpreted as phagosomes containing a fine granular and fibrilar material similar to the slime layer of K rhinoscleromatis, which was also present at times within these vesicles. The ultrastructure of the microorganism is illustrated. The limiting membrane of the phagosomes was frequently ruptured and fragmented, resulting in the close contact of the bacteria and their slime layer with the cytoplasm. Some phagosomes contained myelin figures and the cellular debris of other cells which consisted of Russell bodies, fragments of polymorphonuclear leukocytes and nuclear debris. In some other cases, the smaller vesicles were considered to be swollen and vacuolated mitochondria. Fusion of vesicles was frequent, forming larger vacuoles and occasional multicellular cysts. The nuclei of the Mikulicz cells were round, oval, or stellate and pyknotic. In the small less altered cells, the chromatin was light and uniformly distributed, with some condensation to the nuclear membrane. Nucleoli were seldom observed. In the large well-developed Mikulicz cell, the nucleus was frequently absent. At times, plasma cells contained cytoplasmic vacuoles that appeared to be dilated rough endoplasmic reticulum. The abundant presence of rough endoplasmic reticulum, the Russell bodies and the peculiar distribution of the nuclear chromatin material were used to differentiate these cells from the Mikulicz cells. Bacteria were never observed in the intact plasma cell. Fragments of plasma cells, debris of other cells and bacteria or slime layer material were seen sometimes loose in the interstitial tissues.
Discussion
Previous investigators have considered the Mikulicz cell as a macrophage, a concept that is confirmed by our findings. The foamy mononuclear cell in rhinoscleroma has all the basic morphologic structures characteristic of macrophages.1″ Although plasma cells may at times appear vacuolated, the distinct ultrastructural features of these cells-ie, abundant rough endoplasmic reticulum, distribution of the nuclear chromatin and presence of Russell bodies-allowed them to be distinguished from macrophages. Bacteria were not seen in plasma cells. The presence of bacteria and mucopolysaccharides in the vacuoles of the Mikulicz cell has been previously reported. As demonstrated in the present study using electron microscopy and fluorescent antibody techniques, the cytoplasmic vacuoles of the Mikulicz cell contained few bacteria but abundant material that was structurally similar to the slime layer of K rhinoscleromatis. This material reacted specifically with fluorescent antibodies directed against the microorganism and was visualized as granules, which probably represent the vacuoles of the Mikulicz cell. Bacteria were more abundant in the microcysts and the interstitium of acutely inflammed areas. An interesting finding, previously mentioned by other investigators in rhinoscleroma, is the frequent fragmentation of the limiting membrane of the phagosomes. Similar disruption of membranes has been observed in leprosy 18 and was interpreted as mechanical rupture resulting from the large numbers of bacteria and their metabolic products present within the phagosomes. In listeriosis.”‘ it has been postulated that this microorganism releases hemolysins containing lecithinase or phospholipase, which are responsible for the lysis of the phagosomal membranes. In the case of other intracellular parasites, the disruption of these membranes has also been demonstrated, but the cause of this rupture is not clearly understood. It is suggested here that in rhinoscleroma the disruption of phagosomal membranes may be mechanical, a result of increased osmotic pressure within the vacuole caused by the mucopolysaccharide of the bacillus. It has been demonstrated in experimental animals and clinical material that macrophages and other cells may become vacuolated and foamy by exposure to nondigestible polysaccharides.”3 Monosaccharides with a molecular weight of up to 200,000 apparently do not produce vacuolization. The slime layer of K rhinoscleromatis is a polysaccharide with an estimated molecular weight of more than 300,000. It is suggested that the bacilli and/or the slime layer penetrate the macrophages by endocytosis and reside in phagosomes that have a membrane which is probably impermeable to these ucopolysaccharides but permeable to water. Water may be drawn inlto the phagosome, resulting in increased intravacuolar pressure, sometimes to the point of rupture and fragmentation of the limiting membrane; if extreme, the cell itself could rupture. A number of vacuoles in the Mikulicz cell appeared to be swollen and vacuolated mitochondria; the cause of this is unknown. Our osmotic theory would explain certain puzzling aspects of this peculiar disease. It would explain the difficulty encountered in attempting to produce experimental lesions with cult-ired microorganisms, since it has been demonstrated that bacilli from artificial culture media have small amounts of the slime layer 12,26 compared with bacilli obtained from human lesions. On the other hand, it is easy to produce antibodies with these cultured bacteria 13,14 and to produce experimental lesions, if adjuvants are added to the inoculum.27 It’would also explain the characteristic prolonged evolution of the disease and the fact that sometimes, even though the histology of the lesions is characteristic, bacteria cannot be grown from them in artificial media. In addition, it would explain the slow regression of the lesions after antibiotic treatment. At present, it is obvious that we cannot omit the possibility of Iysosomal enzyme inhibition or deficiency in these macrophages. The role of plasma cells and specific circulating antibodies in the pathogenesis of rhinoscleroma and the peculiar tendency to produce abundant collagen remain to be ascertained.